С этого конспекта написанного в начале 2016 года начался собственный, независимый разбор работы ферментов, поскольку только на собственных изысканиях можно нормально моделировать, если просто читать учебники то будет как в анекдоте про жигули — постоянно будет одно и тоже получаться. Никакие турбо курсы моделированию вас не научат. Писался этот конспект в обеденные перерывы и паузы на работе, порядка 1 месяца.
Купила компания Мерседес завод АвтоВАЗ.
Перенастроили производство, запускают конвейер…
Бац! на выходе Жигули!Демонтируют оборудование, пригнали новое из Германии, установили,
наладили, запускают.
!!! снова Жигули!Увольняют нахер весь персонал завода, привозят работников из Германии,
налаживают, проверяют, запускают.
СЦУКО! На выходе вновь — Жигули!Около завода холм, на нём отдыхают главный инженер и директор завода
(оба с приставкой «экс»). Смотрят на всё это.
Инженер директору:
— А я тебе говорил — место проклятое! А то всё «руки из жопы, руки из
жопы»…
Надо простыми рассуждениями отбросить все несущественные детали.
- Основа работоспособности клетки — пути синтеза и потребления АТФ, а не метаболиты появляющиеся при его дефиците, не надо с ними бороться и много времени тратить на их изучение.
- Главный лимитер, почему падает мощность клеток до 40-45% от МАМ — креатинфосфатный шаттл, который не тренируется, лишь принудительное повышение синтеза фермента КФК и гиперэкспрессия PGC-1A может его улучшить и сдвинуть выше этот %. В теории можно достичь уровня и 70% от МАМ.
- Митохондрии как просто объем не лимитируют производительность.
- Раз все производят +/-10 ватт одинаковые ватты с 1 литра кислорода при кручении велосипеда, экономичность, утечка электронов из дыхательной цени, белки разобщения UCP2/3 не играют никакой роли, это в лучшем случае 2-3%, экономичность идет от рычагов, точек крепления мышц, маленького веса тела и ног, техники. Ничто из этого не делает выдающегося результата само по себе.
- Практически все ферменты гликолиза снижаются по мере роста квалификации стайера, роль их кол-ва равна НУЛЮ у здоровых людей без мутаций и болезней. Гликолиз производит очень мало АТФ, и просто-напросто при гипотетической работе на 100% от гликолиза мышц весь запас гликогена кончится за 2-3 минуты. Замеры скорости потребления лактата в покое печенью, скорости ресинтеза гликогена и переноса лактата и глюкозы через мембраны показывают что никогда на лету лактат не сможет успевать возвращаться в клетку в виде глюкозы. Гипотетическая 20х скорость работы анаэробного гликолиза бессмысленна по тому что его невозможно обеспечить субстратом. В итоге вклад АТФ из гликолиза 3-5%, а успех спринтеров связан с мышечной массой и… выносливостью, вся основная АТФ из запаса КрФ (который только от МХ ресинтезируется) и митохондрии напрямую АТФ дают.
- Аспартат-малатный и глицероловый шаттлы переносят 1.5 и 2.5 АТФ из гликолиза, и то что аспартат-малатный немного реагирует на тренировки не важно, вклад все равно слишком мал, хотя есть статьи в которых на их работу отводят больше синтеза АТФ при мышечной работе.
- Способность эритроцитов переносить лактат и H+ конечна, избыток ROS разрушает клетки крови, пассивные мышцы не крутят цикл Кребса, так что не будем вовсе рассматривать в этой части все механизмы все клетки и буферные системы тоже.
- Цикл Кребса у нетренированного человека не накапливает избытка продуктов, следовательно возможно он не лимитирует. После тренировок ферментов становится ещё больше и промежуточных продуктов ещё меньше.
- Наступление АэП не от дефицита митохондрий и/или их способности синтезировать АТФ, а от недостаточной доставки жиров внутрь митохондрий, что активирует сначало АнГ а затем и аэробный гликолиз. Клетки между АэП и АнП могут вполне покрывать все потребности в АТФ себя за счет дыхания митохондрий.
- Активизация сигнальных каскадов жирового обмена ведет к росту всех белков помогающих переносить и окислять жиры, может так-же подавлять синтез ферментов гликолиза. НО! Эти сигналы могут и не повышать способность митохондриальной сети производить АТФ. Рост АэП может быть как следствием эффективных тренировок всей мышцы и спутником роста АнП, а может и вообще ничего не значить и не быть связанным с ростом результата если это единственная адаптация.
- Наступление АнП от того что на данном каденсе митохондрия не может полностью обеспечить АТФ клетку, но во всех более высокопороговых клетках есть митохондрии.
Цель данного конспекта была
- Составить список какие ферменты реагируют на HIIT (высокоинтенсивные интервальные тренировки, спринты),
- Какие режимы интервалов дают лучшее воздействие на разные ферменты
- В какой период идет де-тренировка ферментов
- Что вызывает их распад.
- Составление таблицы воздействие/фермент
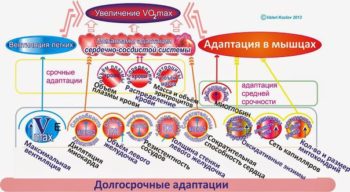
Сроки тренировки и детренировки разных систем
Визуальное отсутствие митохондрий — а в реальности они есть. https://1belok.ru/agogee/malye-stati/mitohondrialnaya-gipertrofiya/
- Пришел к выводу что в первую очередь надо смотреть на рекрутирование мышц (которое не измеряют в этих исследованиях в 95% случаев), важно не ЧТО, а ГДЕ, а детали биохимические не нужны спортсменам.
- Попытки «пересказать своими словами» ( аля «вот тут у нас бензопровод узкое звено») работу ферментов бесполезны в виду того что спортсмен ферменты не измеряет. Надо мерить результат соревнований.
- Я нашел данные о 20+ ферментах реагирующих на HIIT, так что можно говорить разве что о смещении акцента на какие-то группы ферментов, или просто использовать как короткие так и длинные ускорения в течении недели для получения максимального воздействия на все типы ферментов.
- Одна из самых важных вещей — ферменты делаются ОЧЕНЬ БЫСТРО (часы/дни + возможность суммации тренировок) , значит весь остальной год надо работать на гипертрофию ММВ и развитие кислородо-транспортной системы.
Проблемы исследований:
- Нет проверки того, реализуется ли сигнал в иРНК а затем в массу белков, и дает ли бОльшая масса исследуемых белков лучше результат соревнований, есть ли связь. А он не всегда реализуется и связи может не быть. Т.е. рост белка произвольного ещё не значит что он был в дефиците до этого.
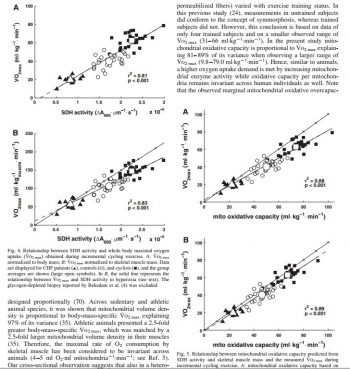
- Не измеряют АэП, АнП, имитацию соревнований не делают, в лучшем случае есть замер МПК, который с той-же цитрат синтазой не коррелирует, МПК коррелирует с гемоглобиновой массой и кислород-транспортом, а не с ферментами мышц! Есть неплохая корреляция МПК с SDH
-
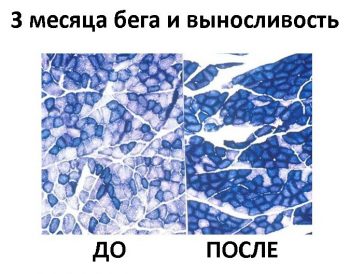
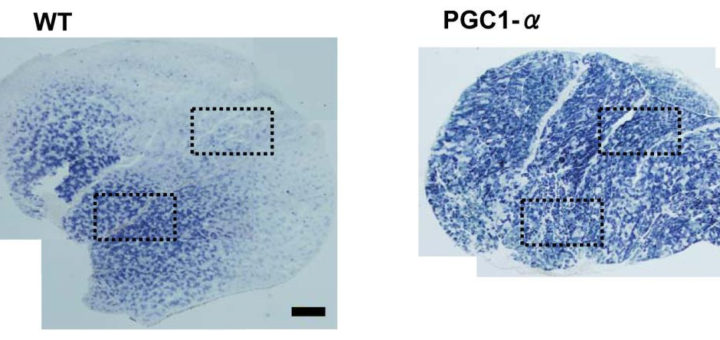
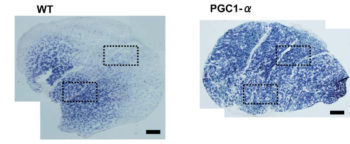
- Нет раздельного замера активности ферментов в ММВ БМВ2А БМВ2Б, о замере внутри каждого волокна речи вообще нет, даже на животных не всегда делают срез всей мышцы. А нужно мерить все строго в каждом мышечном волокне, примерно как тут, где гиперэксперссия PGC-1a в изначально богатых succinate dehydrogenase волокнах дала +62.7%, а в дальних, пустых аж +442.1% (в целом цифры уровня от 3-5 до 7-12 раз разницы по тем или иным замерам различают невыносливую мышечную клетку от предельно выносливой элитного стайера), если же мерить среднее то вышла бы неинформативная цифра не отражающая критически важную суть ГДЕ происходил рост ферментов, не надо среднюю по больнице, надо строго конкретно, а этого практически нигде не делают. Аналогично капилляризацию, миоглобин и все остальное надо измерять отдельно во всех двигательных еденицах. Все рассуждения о «объемах» слабо показывают с чего это высокопороговые ДЕ начнут на них реагировать, кроме истощения гликогена ещё никто не привел механизмов этого воздействия. Конечно существует обмен сигналами и даже обмен митохондриями между клетками, но нет улик что этого может хватить на серьезные сдвиги в ВПДЕ. На картинке вся мышцы крысы.
- Измеряют всего 1-2-3 фермента вместо всех существующих, многие минимально исследованы в разрезе разных типов интервалов.
- Нет контроля гипертрофии, не измеряют капилляризацию и гемоглобиновую массу, другие структуры. В итоге измеряют одно, а рост может быть вообще от другого, от событий вообще не в мышечных клетках!
- Нет индивидуальных результатов. У каждого рост может быть по своей причине! Или его отсутствие, данные о подопытных минимальны, нет развернутой медицинской карты, замера силы, гипертрофии. В итоге 2-3 человка увеличивают свой АнП на 100 ватт, остальные на 20 ватт, всех их делят.
- Методы оценки активности ферментов не воспроизводят их комплексную работу в живой клетке, роль мембран, расположения, не моделируют взаимодействие между клетками, митохондриями, нужно видео с подсветкой разными цветами всех ферментов из живой клетки внутри мышц во время реальной работы, без биопсий, и по динамике живой клетки надо оценивать реакцию на тренировку.
- В итоге, исследования где дают более менее развернутую картинку — единичные, а остальные никакой пользы кроме того что фермент N может реагировать на HIIT/спринты не несут.
- Необходимо, но не достаточно. Так можно сказать о большинстве данных от исследователей. Как следствие их данные имеют низкое качество и нулевой практический смысл. А что же реально надо измерять? Сами соревнования и надо измерять, без промежуточных звеньев по ним оценивать эффективность тренировочных воздействий, делая прикидки/проходки периодически.
- В итоге качественные исследования где есть широкий срез являются единичными. Пример исследования с более-менее качественной работой, и то не идеал. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4552518/
Были отсмотрены следующие ключевые слова
- Ферменты окислительного фосфорилирования (OXPHOS)
- Ферменты гликолиза
- Дыхательная цепь переноса электронов (слово Complex дает слишком много шумной информации без него искал)
- Цикл Кребса
Было найдено что реагируют на спринты/интервалы следующие ферменты (выделено жирным шрифтом):
(«interval training»[Title/Abstract] OR «intensified training» OR HIIT[Title/Abstract] OR HIT[Title/Abstract] OR sprint[Title/Abstract] OR all-out[Title/Abstract] OR wingate[Title/Abstract]) AND ( «Aconitase» OR «Isocitrate dehydrogenase» OR «α-Ketoglutarate dehydrogenase» OR «Succinyl-CoA synthetase» OR «Fumarase» OR «Malate dehydrogenase» OR NADH OR NAD OR «pyruvate dehydrogenase kinase» OR PDK OR «3-hydroxy-acyl-CoA dehydrogenase» OR LDH OR «lactate dehydrogenase» OR «hexokinase» OR «phosphofructokinase» OR «citrate synthase» OR «succinate dehydrogenase» OR «malate dehydrogenase» OR «cytochrome» OR «β-hydroxyacyl-CoA dehydrogenase» OR «β-HAD» OR «aconitate» OR «isocitrate» OR «oxoglutarate» OR «malate» OR «fumarate» OR «oxaloacetate» OR «succinyl» OR «succinate» OR «SDHA» OR «SDHB» OR «SDHC» OR «SDHD» OR «SUCLG1» OR «SUCLG2» OR «fumarase» OR «OGDH» OR «DLST» OR «DLD» OR «IDH3A» OR «IDH3B» OR «IDH3G» OR «ACO2» OR «Coenzyme» OR «CoA» OR «HSCoA» OR «CoASH» OR «ubiquinone» OR «ubidecarenone» OR «Malate dehydrogenase» OR «MDH2» OR «Acetyl-CoA» OR «PFK» OR «Phosphofructokinase» OR «Glucose 6-phosphate» OR «G6P» OR «Fructose 6-phosphate» OR «F6P» OR «Glyceraldehyde 3-phosphate» OR «G3P» OR «GA3P» OR «GADP» OR «Dihydroxyacetone phosphate» OR «DHAP» OR «Glyceraldehyde 3-phosphate dehydrogenase» OR «GAPDH» OR «Bisphosphoglycerate» OR «PGAP» OR «1,3BPG» OR «1,3-Bisphosphoglyceric acid» OR «3-Phosphoglyceric acid» OR «3PG» OR «hexokinase» OR «Fructose-bisphosphate» OR «aldolase» OR «Fructose 1,6-bisphosphate» OR «Triosephosphate isomerase» OR «TPI» OR «TIM» OR «Phosphoglycerate kinase» OR «PGK» OR «Phosphoglycerate mutase» OR «PGM» OR «Enolase» OR «phosphopyruvate hydratase» OR «2-Phosphoglyceric acid» OR «2-phosphoglycerate» OR «2PG» OR «Phosphoenolpyruvic acid» OR «PEP» OR «Pyruvate kinase» OR «Aspartic acid» OR «LDHA» OR «Lactate dehydrogenase» OR «PKM2» OR «Pyruvate kinase» OR «Aldolase» OR «ALDOC» OR «PFKM» OR «6-phosphofructokinase» OR «Glucose-6-phosphate» OR «isomerase» OR «GPI» OR «GLUT1» OR «GLUT4» OR «SDHA» OR «SDHB» OR «SDHC» OR «SDHD» ) NOT rat NOT disease NOT COPD NOT doms NOT inflammation NOT cancer NOT damage NOT inflammatory NOT supplementation NOT bacteria NOT lymphoma
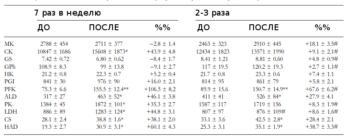
Хорошая табличка о реакции ферментов на HIIT
Число упоминаний ключевых слов:
- citrate synthase +43
- Phosphofructokinase PFK +20
- LDH lactate dehydrogenase +15
- cytochrome (COX cytochrome c oxidase) +10
- Aconitase -1, важно
- GLUT4 +6
- Malate dehydrogenase MDH +5
- Hexokinase +3
- succinate dehydrogenase +2
- malate dehydrogenase +3
- pyruvate dehydrogenase kinase PDK +1
- Pyruvate kinase +2
- PDK4 +2
- 3-hydroxy-acyl-CoA dehydrogenase +1
- β-hydroxyacyl-CoA dehydrogenase +1
- OGDH -1 снижается
- Acetyl-CoA acetyl-CoA carboxylase +2
- Glucose 6-phosphate +2
- GAPDH +1
- Phosphoglycerate kinase PGK +1
- Aldolase +1
- Coenzyme 2 ?
- Isocitrate dehydrogenase 0
- α-Ketoglutarate dehydrogenase 0
- Succinyl-CoA synthetase 0
- Fumarase 0
- NADH 0
- NAD 0
- β-HAD 0
- aconitate 0
- isocitrate 0
- oxoglutarate 0
- malate 0
- fumarate 0
- oxaloacetate 0
- succinyl 0
- succinate 0
- SDHA 0
- SDHB 0
- SDHC 0
- SDHD 0
- SUCLG1 0
- SUCLG2 0
- fumarase 0
- DLST 0
- DLD 0
- IDH3A 0
- IDH3B 0
- IDH3G 0
- ACO2 0
- CoA
- HSCoA
- CoASH
- ubiquinone 0
- ubidecarenone 0
- MDH2 0
- G6P
- Fructose 6-phosphate 0
- F6P
- Glyceraldehyde 3-phosphate 0
- G3P 0
- GA3P 0
- GADP 0
- Dihydroxyacetone phosphate 0
- DHAP 0
- Glyceraldehyde 3-phosphate dehydrogenase 0
- Bisphosphoglycerate 0
- PGAP 0
- 1,3BPG 0
- 1,3-Bisphosphoglyceric acid 0
- 3-Phosphoglyceric acid 0
- 3PG 0
- hexokinase 0
- Fructose-bisphosphate 0
- Fructose 1,6-bisphosphate 0
- Triosephosphate isomerase 0
- TPI 0
- TIM 0
- Phosphoglycerate mutase PGM 0
- Enolase 0
- phosphopyruvate hydratase 0
- 2-Phosphoglyceric acid 0
- 2-phosphoglycerate 2PG 0
- Phosphoenolpyruvic acid 0
- PEP 0
- Aspartic acid 0
- LDHA 0
- PKM2 0
- ALDOC 0
- PFKM 0
- 6-phosphofructokinase 0
- isomerase 0
- GPI 0
- GLUT1 0
Дет-тренировка и ферменты https://vk.com/wall-73104052_4120
https://vk.com/wall-73104052_2109
https://vk.com/wall-73104052_2320
https://vk.com/wall-73104052_2344
Исследования где смотрели отдельно адаптацию 2 типа мышечных волокон:
- http://dx.doi.org.sci-hub.cc/10.1111/j.1600-0838.2010.01136.x
- http://jap.physiology.org/content/101/3/721.long
- http://www.ncbi.nlm.nih.gov/pubmed/7665396
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3912323/
- http://www.ncbi.nlm.nih.gov/pubmed/24260508
- http://www.ncbi.nlm.nih.gov/pubmed/12736837
- http://jap.physiology.org/content/115/5/723.long
- Сила отдельных волокон http://www.ncbi.nlm.nih.gov/pubmed/8770009
- http://www.ncbi.nlm.nih.gov/pubmed/24706192
- http://www.ncbi.nlm.nih.gov/pubmed/2361892
- http://ptjournal.apta.org/content/73/10/661.long
- http://www.ncbi.nlm.nih.gov/pubmed/2745335
- http://jap.physiology.org/content/86/3/915.long
- http://www.ncbi.nlm.nih.gov/pubmed/25211703
- Не-инвазивный метод, корреляция карнозина и доли БМВ http://www.ncbi.nlm.nih.gov/pubmed/21760934
- http://www.ncbi.nlm.nih.gov/pubmed/134623
- http://www.ncbi.nlm.nih.gov/pubmed/24550842
- http://jap.physiology.org/content/90/6/2212.long
- http://www.ncbi.nlm.nih.gov/pubmed/26575622
- http://dx.doi.org.sci-hub.cc/10.1002/mrm.24168
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3749530/
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4552518/
- http://www.ncbi.nlm.nih.gov/pubmed/26183484
- http://www.ncbi.nlm.nih.gov/pubmed/24833978
- http://www.ncbi.nlm.nih.gov/pubmed/24736598
- http://www.ncbi.nlm.nih.gov/pubmed/25268477
- http://www.ncbi.nlm.nih.gov/pubmed/25326514
- http://www.ncbi.nlm.nih.gov/pubmed/26152692
- http://www.ncbi.nlm.nih.gov/pubmed/26247789
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4332652/
Вибрация+ХИТ http://www.ncbi.nlm.nih.gov/pubmed/25679998
ГИпертрофия от ХИТ http://www.ncbi.nlm.nih.gov/pubmed/25395872
Конкурент усиливает эффект http://www.ncbi.nlm.nih.gov/pubmed/26885978
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741131/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4047011/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741131/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178290/
http://www.ncbi.nlm.nih.gov.sci-hub.cc/pubmed/21883960
http://www.ncbi.nlm.nih.gov/pubmed/1918374
http://www.ncbi.nlm.nih.gov/pubmed/26121248
http://www.ncbi.nlm.nih.gov/pubmed/24770423
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741131/
Полезные на мой взгляд данные:
- Важное явление — мощность в ваттах на 1 литр кислорода производимая людьми на велоэргометре никак не зависит от стажа тренировок и разнится +/- 5%. Это ставит под вопрос смысл повышения каких-либо ферментов выше уровня который есть у нетренированных людей в ДЕ на уровне АнП, а этот рост существует даже в НПДЕ от тренировок. Повышать надо активную мышечную массу в первую очередь. http://sci-hub.cc/10.1007/BF01466278#
- 14 тренировок за 2 недели, или за 6 недель с 2 днями отдыха. Те кто чаще делал 30 сек спринты во всю получили больше ферментов, при полном отсутствии роста мощности в тесте (ватты во время этого-же 30 сек теста), те кто отдыхал получил ферментов меньше, зато результ вингейт теста вырос (я трактую как то что они получили гипертрофию мышц и не перетренировались) The volunteers performed a 30-s supramaximal cycling test on a cycle ergometer before and after training. Muscle biopsies were obtained from the vastus lateralis before and after each test to examine metabolites and enzyme activities. Both training programmes led to a marked increase (all significant, P < 0.05) in enzymatic activities related to glycolysis (phosphofructokinase — SP 107%, LP 68% and aldolase — SP 46%, LP 28%) and aerobic metabolism (citrate synthase — SP 38%, LP 28.4% and 3-hydroxyacyl-CoA dehydrogenase — SP 60%, LP 38.7%). However, the activity of creatine kinase (44%), pyruvate kinase (35%) and lactate dehydrogenase (45%) rose significantly (P < 0.05) only in SP. At the end of the training programme, SP had suffered a significant decrease in anaerobic ATP consumption per gram muscle (P < 0.05) and glycogen degradation (P < 0.05) during the post-training test, and failed to improve performance. In contrast, LP showed a marked improvement in performance (P < 0.05) although without a significant increase in anaerobic ATP consumption, glycolysis or glycogenolysis rate. These results indicate that high-intensity cycling training in 14 sessions improves enzyme activities of anaerobic and aerobic metabolism. These changes are affected by the distribution of rest periods, hence shorter rest periods produce larger increase in pyruvate kinase, creatine kinase and lactate dehydrogenase. However, performance did not improve in a short training programme that did not include days for recovery, which suggests that muscle fibres suffer fatigue or injury.xliv
- При тренировках 3 раза в неделю с днями отдыха, когда тренировки на 70% от МПК и интервалы иcпользовались в один день через 1 час отдыха, либо были ежедневные тренировки лучший рост ферментов, выше гликоген и выше окисление жиров получают те кто делает дни отдыха, но результат имитации соревнований вышел одинаковый. Seven endurance-trained cyclists/triathletes trained daily (High) alternating between 100-min steady-state aerobic rides (AT) one day, followed by a high-intensity interval training session (HIT; 8 x 5 min at maximum self-selected effort) the next day. Another seven subjects trained twice every second day (Low), first undertaking AT, then 1-2 h later, the HIT. These training schedules were maintained for 3 wk. Forty-eight hours before and after the first and last training sessions, all subjects completed a 60-min steady-state ride (60SS) followed by a 60-min performance trial. Muscle biopsies were taken before and after 60SS, and rates of substrate oxidation were determined throughout this ride. Resting muscle glycogen concentration (412 +/- 51 vs. 577 +/- 34 micromol/g dry wt), rates of whole body fat oxidation during 60SS (1,261 +/- 247 vs. 1,698 +/- 174 micromol.kg(-1).60 min(-1)), the maximal activities of citrate synthase (45 +/- 2 vs. 54 +/- 1 mmol.kg dry wt(-1).min(-1)), and beta-hydroxyacyl-CoA-dehydrogenase (18 +/- 2 vs. 23 +/- 2 mmol.kg dry wt(-1).min(-1)) along with the total protein content of cytochrome c oxidase subunit IV were increased only in Low (all P < 0.05). Mitochondrial DNA content and peroxisome proliferator-activated receptor-gamma coactivator-1alpha protein levels were unchanged in both groups after training. Cycling performance improved by approximately 10% in both Low and High. We conclude that compared with training daily, training twice every second day compromised high-intensity training capacity. While selected markers of training adaptation were enhanced with twice a day training, the performance of a 1-h time trial undertaken after a 60-min steady-state ride was similar after once daily or twice every second day training programs.i
- ЭМГ более информативный маркер перетренировки у лошадей от избытка интенсивных нагрузок чем замеры ферментов, они не отражают падение ЭМГ-активности. Compared with NT controls, IT induced a stronger adaptation (e.g., higher amplitude, shorter duration, and fewer turns) in QEMG variables resembling potentially synchronization of individual motor unit fiber action potentials. Despite a 19% decrease in performance of the SET after IT, enzyme activities of 3-hydroxyacyl dehydrogenase and citrate synthase displayed similar increases in control and IT animals. We conclude that 1) QEMG analysis is a more sensitive tool to monitor training adaptation than muscle enzyme activities but does not discriminate between overreaching and normal training adaptations at this training level and 2) the decreased performance as noted in this study after IT originates most likely from a central (brain) rather than peripheral level.ii
- Совмещение спринтов и силовой тренировки на 1 тренировке может мешать сигналам клеточным. Мой комментарий — не надо делать по их схемам, отдыхать больше надо и меньше подходов. We examined acute molecular responses in skeletal muscle to repeated sprint and resistance exercise bouts. Six men [age, 24.7 +/- 6.3 yr; body mass, 81.6 +/- 7.3 kg; peak oxygen uptake, 47 +/- 9.9 mlxkg(-1)xmin(-1); one repetition maximum (1-RM) leg extension 92.2 +/- 12.5 kg; means +/- SD] were randomly assigned to trials consisting of either resistance exercise (8 x 5 leg extension, 80% 1-RM) followed by repeated sprints (10 x 6 s, 0.75 Nxm torquexkg(-1)) or vice-versa. Muscle biopsies from vastus lateralis were obtained at rest, 15 min after each exercise bout, and following 3-h recovery to determine early signaling and mRNA responses. There was divergent exercise order-dependent phosphorylation of p70 S6K (S6K). Specifically, initial resistance exercise increased S6K phosphorylation ( approximately 75% P < 0.05), but there was no effect when resistance exercise was undertaken after sprints. Exercise decreased IGF-I mRNA following 3-h recovery ( approximately 50%, P = 0.06) independent of order, while muscle RING finger mRNA was elevated with a moderate exercise order effect (P < 0.01). When resistance exercise was followed by repeated sprints PGC-1alpha mRNA was increased (REX1-SPR2; P = 0.02) with a modest distinction between exercise orders. Repeated sprints may promote acute interference on resistance exercise responses by attenuating translation initiation signaling and exacerbating ubiquitin ligase expression. Indeed, repeated sprints appear to generate the overriding acute exercise-induced response when undertaking concurrent repeated sprint and resistance exercise. Accordingly, we suggest that sprint-activities are isolated from resistance training and that adequate recovery time is considered within periodized training plans that incorporate these divergent exercise modes.iii
- Тренировки по 30 секунд на 184% от МПК (643 +/- 22 W ) либо 20 минутые отрезки на 87% от МПК (304 +/- 12 W ) у элитных велосипедистов дают практически одинаковые сигналы, но от коротких спринтов немного более высокие уровни PDK4 PRC Tfam NRF-2. The mRNA of upstream markers of mitochondrial biogenesis [peroxisome proliferator-activated receptor-γ coactivator-1 (PGC-1α), PGC-1α-related coactivator (PRC) and peroxisome proliferator-activated receptor δ (PPARδ)] increased to the same extent after SIE and IE (6-, 1.5- and 1.5-fold increase, respectively). Of the downstream targets of PGC-1α, mitochondrial transcription factor A (Tfam) increased only after SIE and was significantly different from that after IE (P < 0.05), whereas others increased to the same extent (pyruvate dehydrogenase kinase, PDK4) or was unchanged (nuclear respiratory factor 2, NRF2). We conclude that upstream genetic markers of mitochondrial biogenesis increase in a similar way in elite athletes after one exercise session of SIE and IE. However, since the volume and duration of work was considerably lower during SIE and since Tfam, the downstream target of PGC-1α, increased only after SIE, we conclude that SIE might be a time-efficient training strategy for highly trained individuals.iv
- Citrate synthase (CS) and β-HAD mRNA were rapidly increased (1 session), followed 2 sessions later (session 3) by increases in CS and β-HAD activities, and mitochondrial DNA. Changes in COX-IV mRNA (session 3) and protein (session 5) were more delayed. Training also increased mitochondrial fission proteins (fission protein-1, >2-fold; dynamin-related protein-1, 47%) and the fusion protein mitofusin-1 (35%) but not mitofusin-2. This study has provided the following novel information: (a) the training-induced increases in transcriptional and mitochondrial proteins appear to result from the cumulative effects of transient bursts in their mRNAs, (b) training-induced mitochondrial biogenesis appears to involve re-modelling in addition to increased mitochondrial content, and (c) the ‘transcriptional capacity’ of human muscle is extremely sensitive, being activated by one training bout.v
- При одинаковых сигналах сразу после тренировки, долгосрочный результат может быть разный. Both protocols elicited similar increases in markers of adenosine monophosphate-activated protein kinase (AMPK) and p38 mitogen-activated protein kinase activation, as well as Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) mRNA expression (main effects for time, P ≤ 0.05). In study 2, we determined whether 6 weeks of the CONT protocol (3 days per week) would increase skeletal muscle mitochondrial content to a similar extent to what we have previously reported after 6 weeks of INT. Despite similar acute signalling responses to the CONT and INT protocols, training with CONT did not increase the maximal activity or protein content of a range of mitochondrial markersvi
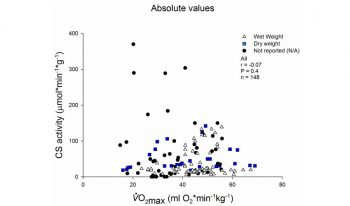
- Попытки оценить работу митохондрий по кинетике потребления О2. Mitochondrial function was assessed with high-resolution respirometry (HRR) and maximal activities of oxidative enzymes citrate synthase (CS) and cytochrome c oxidase (COX) were accordingly determined. In response to HIT, V̇O2 kinetics became faster (τ: 20.4 ± 4.4 vs. 28.9 ± 6.1 s; P<0.01) and fatty acid oxidation (ETFP) and leak respiration (LN) both became elevated (P<0.05). Activity of CS and COX did not increase in response to training. Both before and after the HIT-period fast V̇O2 kinetics (low τ values) was associated with large values for ETFP, electron transport system capacity (ETS) and electron flow specific to complex II (CIIP) (P<0.05). Collectively these findings support that selected measures of mitochondrial function obtained with HRR are important for fast V̇O2 kinetics and better markers than maximal oxidative enzyme activity in describing the speed of the V̇O2 response during moderate intensity exercise.vii
- Тренировки на низких углеводах могут улучшить результат тренированных спортсменов, не затронув уровни цитрат синтазы и других ферментов. The training-induced improvement in 250-kJ time trial performance was greater (p = .02) in the HI-LO group (211 ± 66 W to 244 ± 75 W) compared with the HI-HI group (203 ± 53 W to 219 ± 60 W); however, the increases in mitochondrial content was similar between groups, as reflected by similar increases in citrate synthase maximal activity, citrate synthase protein content and cytochrome c oxidase subunit IV protein content (p > .05 for interaction terms). This is the first study to show that a short-term «train low, compete high» intervention can improve whole-body exercise capacity. Further research is needed to determine whether this type of manipulation can also enhance performance in highly-trained subjects.viii
- Ферменты окислительного фосфорилирования в БМВ и кинетика О2 велосипедистов не увеличились от высокоинтенсивных тренировок. Content of CS, COX-4, and PFK in homogenate and fast-twitch fibers was unchanged with HIT. Maximal activity (μmol g DW(-1) min(-1)) of CS (56 ± 8 post-HIT vs. 59 ± 10 pre-HIT), HAD (27 ± 6 vs. 29 ± 3) andPFK (340 ± 69 vs. 318 ± 105) and the capillary to fiber ratio (2.30 ± 0.16 vs. 2.38 ± 0.20) was unaltered following HIT. V˙O2 kinetics was unchanged with HIT and the speed of the primary response did not differ between MOD and INT. Muscle creatine phosphate was lower (42 ± 15 vs. 66 ± 17 mmol kg DW(-1)) and muscle lactate was higher (40 ± 18 vs. 14 ± 5 mmol kg DW(-1)) at 6 min of INT (P < 0.05) after compared to before HIT. A period ofintensified training with a volume reduction did not increase the content of oxidative enzymes in fast-twitch fibers, and did not change V˙O2 kinetics.ix
- Введение dichloroacetate крысам снижает уровни лактат в мышцах и крови, практически не затрагивает уровни РНК, но в долгосрочной перспективе снижает уровни митохондриальных маркеров. We next examined the effects of acute DCA administration on mRNA expressions involved with mitochondrial biogenesis after same high-intensity interval exercise and the effects of chronic DCA administration on mitochondrial adaptations after high-intensity interval training (increasing intensity from 38 to 43 m/min by the end of training period). Acute DCA administration did not change most of the exercise-induced mRNA upregulation. These data suggest that lactate reductions by DCA administration did not affect transcriptional activation after high-intensity interval exercise. However, chronic DCA administration attenuated, in part, mitochondrial adaptations such as training-induced increasing rates of citrate synthase (P = 0.06), β-hydroxyacyl CoA dehydrogenase activity (P < 0.05), cytochrome c oxidase IV (P < 0.05) and a fatty acid transporter, fatty acid translocase/CD36 (P < 0.05), proteins after exercise training. These results suggest that lactate accumulation during high-intensity interval exercise may be associated with mitochondrial adaptations after chronic exercise training.x
- Масса митохондрий (по маркерам цитрат синтазы) и их респирация не коррелируют в данном исследовании, лучшая респирация была от коротких 30 секундных спринтов во всю. PGC-1a, p53, and PHF20 более информативны чем цитрат синтаза для оценки риспирации митохондрий. The maximal mitochondrial respiration in permeabilized muscle fibers increased significantly only after SIT (25%). Similarly, the protein content of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α, p53, and plant homeodomain finger-containing protein 20 (PHF20) increased only after SIT (60-90%). Conversely, citrate synthase activity, and the protein content of TFAM and subunits of the electron transport system complexes remained unchanged throughout. Our findings suggest that training intensity is an important factor that regulates training-induced changes in mitochondrial respiration and that there is an apparent dissociation between training-induced changes in mitochondrial respiration and mitochondrial content. Moreover, changes in the protein content of PGC-1α, p53, and PHF20 are more strongly associated with training-induced changes in mitochondrial respiration than mitochondrial contentxi
- Тренировки нарушают аконитазу в трицепсе и не ведут к адаптации, а вот в квадрицепсе это компенсируется массой митохондриальных белков. Возможно цитрат играет роль в этом процессе. Чрезмерное закисление рушит адаптацию. Возможно период легких тренировок для трицепса предотвратит это явление. In this study, we examined biopsy specimens of vastus lateralis and triceps brachii in healthy volunteers, together with primary human myotubes. An intense exercise regimen inactivated aconitase by 55-72%, resulting in inhibition of mitochondrial respiration by 50-65%. In the vastus, the mitochondrial dysfunction was compensated for by a 15-72% increase in mitochondrial proteins, whereas H2O2 emission was unchanged. In parallel with the inactivation of aconitase, the intermediary metabolite citrate accumulated and played an integral part in cellular protection against oxidative stress. In contrast, the triceps failed to increase mitochondrial density, and citrate did not accumulate. Instead, mitochondrial H2O2 emission was decreased to 40% of the pretraining levels, together with a 6-fold increase in protein abundance of catalase. In this study, a novel mitochondrial stress response was highlighted where accumulation of citrate acted to preserve the redox status of the cell during periods of intense exercise.xii
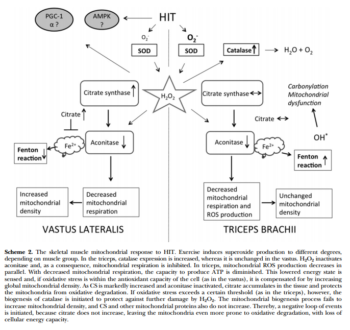
Информация ниже особого смыла не несет, кроме списка ферментов которые реагируют на спринты:
- Mg2+ stimulated ATPase, myokinase and creatine phosphokinase increased 30, 20, and 36 percentxiii
- Aside from differences in fiber composition and enzymes among middle-distance runners, the only distinction between the sexes was the larger fiber areas of the male athletes. SDH activity was found to correlate 0.79 with VO2max, while muscleLDH appeared to be a function of muscle fiber composition. While sprint- and endurance-trained athletes are characterized by distinct fiber compositions and enzyme activities, participants in strength events (e.g., shot-put) have relatively low muscle enzyme activities and a variety of fiber compositions.xiv
- Although muscle fibre composition did not change a pronounced muscle adaptation took place with the training with enhancement of the SDH activity of the S and E legs while the NT-leg did not change. Blood flow and oxygen uptake were similar in NT and S-E legs while femoral vein oxygen content was slightly lower in the trained as compared to the NT-legxv
- Significant mean differences (P less than 0.05) between Groups A and B were found for VO2 max (67 and 57 ml/kg/min), malate dehydrogenase (MDH) and phosphorylase (PH), in biopsied muscle. No differences were evident between Groups A and B for % slow twitch (ST) and % fast twitch (FT) fibers, or in area FT or ST. Nor was there any difference in the mean activities of succinate dehydrogenase (SDH) and lactate dehydrogenase (LDH) between the groups. Significant correlations were found between VO2 max and SDH, VO2 max and MDH, and between SDH and MDH. These data also indicate that an extremely high percentage of FT or ST fibers may not be a requirement for success in competitive cycling as has been found in earlier studies on sprint or endurance running.xvi
- Endurance training resulted in significant increases in VO2max (58.4 to 64.3 ml . min-1 . kg-1), in ST and FTa fiber area (6.0 to 7.3 and 8.0 to 10.4 microns 2 x 10(3), respectively), and in SDH activity (6.4 to 9.1 IU). After detraining VO2max and SDH activity returned to pretraining levels. Sprint training resulted in a significant increase only in PFK activity (28.1 to 33.9 IU), which was also abolished in the detraining periodxvii
- Before and after the 5-week training period, muscle biopsies were taken out of the lateral head of m. gastrocnemius and analyzed for the activities of phosphorylase, phosphofructokinase (PFK), glyceraldehyde phosphate dehydrogenase (GAPDH), lactate dehydrogenase (LDH), succinate dehydrogenase (SDH), and malate dehydrogenase (MDH). Following training there was a significant increase in the subjects’ performance time in a treadmill test at a speed of 16 km/h speed and 15% grade. Significant increases were observed in the activities of phosphorylase, PFK, GAPDH, LDH, and MDH, whereas the 17.5% increase in SDH was not statistically significant. It is concluded that interval training with high intensity and a 1:4 work-rest ratio leads to increased activities of key enzymes involved in glycogenolysis and anaerobic glycolysis of skeletal muscle.xviii
- OGDH (P less than 0.05) and decreased fiber type IIb proportion and the PFK/OGDH ratio. No significant change was observed for CK, HK, PFK, and LDH. Large interindividual differences in the response to training were observed for all variables. However, intraclass correlations indicated that the extent of the response of ALC and CK, HK, LDH, MDH, and OGDHactivities and of the PFK/OGDH activity ratio to training were significantly similar within pairs of twins. Although the role of heredity appeared absent for the changes in fiber type proportions and in anaerobic alactacid capacity, the present results suggest that the response of anaerobic lactacid capacity and most enzyme activities to high-intensity intermittent training is significantly determined by the genotype.xix
- Biopsy samples were obtained from vastus lateralis of eight female subjects before and after a maximal 30-s sprint on a nonmotorized treadmill and were analyzed for glycogen, phosphagens, and glycolytic intermediates. Peak power output averaged 534.4 +/- 85.0 W and was decreased by 50 +/- 10% at the end of the sprint. Glycogen, phosphocreatine, and ATP were decreased by 25, 64, and 37%, respectively. The glycolytic intermediates above phosphofructokinase increased approximately 13-fold, whereas fructose 1,6-diphosphate and triose phosphates only increased 4- and 2-fold. Muscle pyruvate and lactate were increased 19 and 29 timesxx
- The mean diameter of each muscle fiber type was significantly higher in the athletes. The mean enzyme activity values in mu kat X g-1 w.w. for cyclists and nonathletes, respectively, were as follows: triosephosphate dehydrogenase (TPDH), 6.2 and 3.78; lactate dehydrogenase (LDH), 4.4 and 4.59; citrate synthase (CS), 0.154 and 0.13; hydroxyacyl-CoA dehydrogenase (HAD), 0.041 and 0.07. The mean difference between groups in TPDH and in (TPDH + LDH)/(CS + HAD) ratio were statistically significant. Maximum voluntary isometric strength (knee extension) was about 17% greater in cyclists than the mean value for Czechoslovakian men of the same age. A strong positive correlation (r = 0.72) between the percent of fast glycolytic fibers (type II B) and isometric strength was observed in the cyclists. Furthermore, mean weight-compensated maximal oxygen consumption (VO2 max, ml X kg-1 X min-1) for all subjects (n = 22) was significantly related to percent of slow oxidative fibers (type I) (r = 0.75) and to the mean diameter of type II B (r = 0.58), fast oxidative-glycolytic fibers (type II A) (r = 0.68) and type I fibers (r = 0.59) xxi
- Approximately 88% was free carnitine, 7% acetylcarnitine and acylcarnitine was estimated at 5%. Exercise did not affect total carnitine, but resulted in a marked fall in free carnitine and almost equivalent rise in acetylcarnitine. The results are consistent with a role for carnitine in the regulation of the acetyl-CoA/CoA ratio during sprint exercise in the Thoroughbred horse by buffering excess production of acetyl units.xxii
- The sum of all glycolytic intermediates from glucose 6-phosphate to pyruvate at exhaustion decreased by a dramatic 80% compared with the 25% decrease for the 10-min fatigue swimming protocol. This large depletion of glycolytic intermediates was accompanied by an 80% fall in ATP, a 70-80% reduction in the ATP/ADP and phosphorylation potential, and a 2.5-fold increase in the NAD/NADH. Associated with these changes was a marked displacement of thephosphoglycerate kinase (PGK), and the combined glyceraldehyde-3-phosphate dehydrogenase-PGK reactions from thermodynamic equilibrium. As a general conclusion, fatigue and exhaustion should be viewed as a multicomponent biochemical process in response to low glycogen and not leveled at one particular step of the glycolytic pathway.xxiii
- citrate synthase and phosphofructokinase activities increasedxxiv
- Nevertheless, their swimming power, sprinting (s.22.86 m-1), endurance (s.365.8 m-1) performance, aerobic capacity, and muscle (m. deltoid) citrate synthase were unchanged as a consequence of the 10-d training regimenxxv
- The citrate synthase activity increased in the gluteus muscle only, and the increase was seen during the first 2 wk. No significant differences were seen in 3-hydroxy-acyl-CoA dehydrogenase and lactate dehydrogenase activities in the muscles during the entire training periodxxvi
- The results show an increase in the percentage of type I fibres and an increase in the diameter of both fibre types. A significant increase was also observed in glycogen content, and in the activities of glycogen synthase, glycogen phosphorylase, phosphofructokinase, pyruvate kinase, succinate dehydrogenase, aspartate aminotransferase and alanine aminotransferase. We conclude that a long period of sprint training induces a biochemical muscle adaptation to anaerobic exercise. This metabolic adaptation is followed by a morphological adaptation, although this is probably not as specific as the biochemical one.xxvii
- Twelve subjects were equally divided into continuous (CT, exercise at 50% maximal work) or interval (IT, 30 s work, 30 s rest at 100% maximal work) training groups that cycled 30 min day-1, 3 days.week-1, for 8 weeks. Following training, aerobic power (VO2max), exercising work rates, and peak power output were all higher (9-16%) after IT than after CT (5-7%). Vastus lateralis muscle citrate synthase activity increased 25% after CT but not after IT. A consistent increase in adenylate kinase activity (25%) was observed only after IT. xxviii
- Citrate synthase activity increased significantly with HIT and decreased significantly with ROTxxix
- This result was suggested in muscle by the increase in lactate production measured after a training session associated with the 20% higher activity of both phosphofructokinaseand lactate dehydrogenasexxx
- The O2 deficit was not related to blood lactate during submaximal exercise, muscle enzyme activity (citrate synthase, 3-hydroxyacyl-CoA-dehydrogenase, lactate dehydrogenase), number of muscle capillaries, %ST fibres or muscle buffer capacity. The accumulated O2 deficit was 36% higher (p < 0.05) during rowing compared to running. The present data suggest that the anaerobic energy production during intense exercise is related to the muscle mass involved. However, it appears that the anaerobic energy turnover is not determined by muscle fibre type distribution, muscle buffer capacity or muscle endurance capacity.xxxi
- Activity of total lactate dehydrogenase (LD) was 33% higher and of M subunit of LD 38% higher in males. Anaerobic performance was directly related to the proportion of type II fibres, the relative M subunit activity or the activity of PFK in both males and females and the higher M subunit activity in males could predict some of the sex difference in anaerobic performancexxxii
- After training, the activity of adenosine 5′-phosphate (AMP) deaminase was lower (P < 0.001) whereas the activities of hypoxanthine phosphoribosyl transferase (HPRT) andphosphofructokinase were significantly higher compared with pre-training levels. The higher activity of HPRT with training suggests an improved potential for rephosphorylation of intracellular hypoxanthine to inosine monophosphate (IMP) in the trained musclexxxiii
- Muscle biopsies from gastrocnemius muscle were obtained before and immediately after R15%,2-3 min, from which muscle lactate and creatine phosphate (CP) concentrations, fibre type distribution, capillaries per fibre, total lactate dehydrogenase (LDH) activity and the LDH isoenzyme pattern were determined. The MOD increased with the treadmill gradient and duration. During both treadmill and track runs, SMDR performance was superior to that of RR, but no significant differences were observed with respect to MOD, muscle fibre type distribution, total LDH activity, its iso-enzyme pattern, changes in muscle lactate or CP concentrations. However, after treadmill runs, peak venous lactate concentration and partial pressures of carbon dioxide were higher, and pH lower in SMDR. Also the number of capillaries per muscle fibre and the maximal oxygen uptake were larger in SMDR. These findings would suggest that the superior performance of SMDR depended more on their aerobic than on their anaerobic capacity.xxxiv
- The subjects also performed a 30 s sprint test on a cycle ergometer. The inter-individual variation in AMP deaminase activity was large, ranging from 5.4 to 27.4 microkat g-1 dry muscle. AMP deaminase was positively correlated with phosphofructokinase (PFK), the marker of the glycolytic capacity of the muscle, but there was no correlation with enzymes of oxidative metabolism, such as 3-hydroxyacyl-CoA dehydrogenase and citrate synthase, or with the activity of myokinase and lactate dehydrogenase. There was no significant correlation between AMP deaminase activity and the proportion of the different muscle fibre types. A direct relationship between AMP deaminase activity on the one hand and glycolytic capacity and sprint performance on the other was found. However, no relationship to oxidative capacity or contractile properties was found.xxxv
- There was an increase in total lactate dehydrogenase (LD) activity following sprint training in both sexes, although the levels were lower in the women both before and after training. Glycogen content increased and the activity of LD iso-enzyme 1 decreased in the women, but not in men. It was hypothesised that both the smaller areas of type II fibres and lower activity of LD generally seen in women may be due in part to less frequent activation of type II fibres in women than in men. If this were the case, the women should respond to sprint training (a type of training that activates type II fibres) to a relatively greater extent than men. That the observed increase in type IIB fibre area in response to sprinttraining was greater in the women than in men supported the hypothesis of the study. However, the results for LD activity, which showed a similar response in the men and the women, did not support the hypothesis.xxxvi
- antioxidant enzymes glutathione peroxidase (GPX), glutathione reductase (GR), and superoxide dismutase (SOD). Activities of several muscle metabolic enzymes were determined to assess the effectiveness of the training. After the first 6-wk training period, no change in GPX, GR, or SOD was observed, but after the 7th week of training there was an increase in GPX from 120 +/- 12 (SE) to 164 +/- 24 mumol.min-1.g dry wt-1 (P < 0.05) and in GR from 10.8 +/- 0.8 to 16.8 +/- 2.4 mumol.min-1.g dry wt-1 (P < 0.05). There was no significant change in SOD. Sprint cycle training induced a significant (P < 0.05) elevation in the activity of phosphofructokinase and creatine kinase, implying an enhanced anaerobic capacity in the trained muscle. The present studyxxxvii
- PPO, time to fatigue at 150% PPO (TF150) and 40-km cycle time trial performance (TT40) all significantly improved afterHIT (P < 0.05). In contrast, there was no change in the activity of either phosphofructokinase or citrate synthase. In addition, beta m correlated significantly with TT40 performance before HIT (r = -0.82, P < 0.05) and the relationship between change in beta m and change in TT40 was close to significance (r = -0.74). beta m did not correlate with TF150. These results indicate that beta m may be an important determinant of relatively short-duration (< 60 min) endurance cycling activity and responds positively to just six sessions of high-intensity, submaximal interval training. demonstrates that intermittent sprint cycle training that induces an enhanced capacity for anaerobic energy generation also improves the level of antioxidant protection in the muscle.xxxviii
- This was associated with a greater glycolytic potential as shown by higher activities for PHOS (9%), PFK (17%) and LDH (31%) after training, without changes in CK and oxidative markers (CS and HAD). Detraining induced significant decreases in VO2peak (4%), MAP (5%) and oxidative markers (10-16%), while Wmax and the anaerobic potential were maintained at a high levelxxxix
- Maximal enzyme activity of hexokinase, phosphofructokinase, citrate synthase, succinate dehydrogenase, and malate dehydrogenase was also significantly (P < 0.05) higher after training. It was concluded that relatively brief but intense sprint training can result in an increase in both glycolytic and oxidative enzyme activity, maximum short-term power output, and VO2 max.xl
- Phosphorylase activity increased (P < 0.025), citrate synthase activity decreased (P < 0.01), but no significant changes were recorded in myokinase and phosphofructokinase activities. The proportion of type II muscle fibres increased significantly (P < 0.05). These results demonstrate that 6 weeks of short sprint training can improve endurance, sprint and repeated sprint ability in fit subjects. Increases in the proportion of type II muscle fibres are also possible with this type of training.xli
- Resting PDH-a with DCA was increased significantly over AC and Con trials (3.58 +/- 0.4 vs. 0.52 +/- 0.1 and 0.74 +/- 0.1 mmol. kg wet muscle(-1). min(-1)). DCA and AC significantly increased resting acetyl-CoA (35.2 +/- 4.4 and 22.7 +/- 2.9 vs. 10.2 +/- 1.3 micromol/kg dry muscle) and acetylcarnitine (12.9 +/- 1.4 and 11.0 +/- 1.0 vs. 3.3 +/- 0.6 mmol/kg dry muscle) over Con. Resting contents of phosphocreatine, lactate, ATP, and glycolytic intermediates were not different among trials. Average power output and total work done were not different among the three 10-s sprint trials. Postexercise, PDH-a in AC and Con trials had increased significantly but was still significantly lower than in DCA trial. Acetyl-CoA did not increase in any trial, whereas acetylcarnitine increased significantly only in DCA. Exercise caused identical decreases in ATP and phosphocreatine and identical increases in lactate, pyruvate, and glycolytic intermediates in all trials. These data suggest that there is an inability to utilize extra oxidative substrate (from either stored acetylcarnitine or increased PDH-a) during exercise at this intensity, possibly because of O(2) and/or metabolic limitations.xlii
- Conversely to normoxia, levels of ATP, ADP and total NADHwere maintained at their resting level under 60% FiO2. These data lead us to suppose a higher oxidation rate for pyruvate and NADH in mitochondria, thereby lowering the metabolic acidosis and allowing a better functioning of the glycolytic and contractile processes to delay the time to exhaustion.xliii
- Sessions consisted of 15-s all-out repetitions with 45-s rest periods, plus 30-sall-out repetitions with 12-min rest periods. The number of repetitions was gradually increased up to a maximum of seven. Biopsy samples of the vastus lateralis muscle were taken before and after training. Performance changes were evaluated by two tests, a 30-s all-out test and a maximal progressive test. Significant increases in phosphocreatine (31%) and glycogen (32%) were found at the end of training. In addition, a significant increase was observed in the muscle activity of creatine kinase (44%), phosphofructokinase (106%), lactate dehydrogenase (45%), 3-hydroxy-acyl-CoA dehydrogenase (60%) and citrate synthase (38%). After training, performance of the 30-s all-out test did not increase significantly, while in the maximal progressive test, the maximum oxygen consumption increased from mean (SD) 57.3 (2.6) ml x min(-1) x kg(-1) to 63.8 (3.0) ml min(-1) x kg(-1), and the maximum load from 300 (11) W to 330 (21) W; all changes were significant. In conclusion, this new protocol, which utilises short durations, high loads and long recovery periods, seems to be an effective programme for improving the enzymatic activities of the energetic pathways in a short period of time.xlv
- AMPK phosphorylates and inhibits acetyl-coenzyme A (CoA) carboxylase (ACC) and enhances GLUT-4 translocation. It has been reported that human skeletal muscle malonyl-CoA levels do not change in response to exercise, suggesting that other mechanisms besides inhibition of ACC may be operating to accelerate fatty acid oxidation. Here, we show that a 30-s bicycle sprint exercise increases the activity of the human skeletal muscle AMPK-alpha1 and -alpha2 isoforms approximately two- to threefold and the phosphorylation of ACC at Ser(79) (AMPK phosphorylation site) approximately 8.5-fold. Under these conditions, there is also an approximately 5.5-fold increase in phosphorylation of neuronal NO synthase-mu (nNOSmu;) at Ser(1451)xlvi
- Our results indicate that, in well-trained individuals, short-term HIT improves metabolic control but does not blunt AMPK signaling in response to intense exercise.xlvii
- In conclusion, these results suggested that the maximal muscle oxidative capacity was related to blood lactate removal ability after a 1-min all-out test. Moreover, maximal muscle oxidative capacity and blood lactate removal ability were associated with the delay in the fatigue observed during continuous and intermittent supramaximal exercises in well-trained subjects.xlviii
- Training resulted in a 7.1% increase in mean power output (p<0.05), an 8% increase in VO2peak (p< 0.001), a 42% increase (p< 0.01) in CS activity and a 17% increase (p< 0.05) in resting intramuscular glycogen content. In contrast, neither PFK activity nor fibre type distribution changed with trainingxlix
- We concluded that skeletal muscle MCT1 expression was associated with the velocity constant of net blood lactate removal after a 1-min all-out test and with the fatigue indexes. It is proposed that MCT1 expression may be important for blood lactate removal after supramaximal exercise based on the existence of lactate shuttles and, in turn, in favor of a better tolerance to muscle fatigue.l
- After SIT, CS maximal activity increased by 38% (5.5 +/- 1.0 vs. 4.0 +/- 0.7 mmol.kg protein(-1).h(-1)) and resting muscle glycogen content increased by 26% (614 +/- 39 vs. 489 +/- 57 mmol/kg dry wt) (both P < 0.05).li
- Except for a modest decrease in phosphofructokinase activity following short-term normobaric hypoxia, no changes were observed in muscle enzyme activities, buffer capacity, capillary density or morphology.lii
- SIT increased muscle glycogen content by approximately 50% (main effect, P=0.04) and the maximal activity of citrate synthase (posttraining: 7.8+/-0.4 vs. pretraining: 7.0+/-0.4 mol.kg protein -1.h-1; P=0.04), but the maximal activity of 3-hydroxyacyl-CoA dehydrogenase was unchanged (posttraining: 5.1+/-0.7 vs. pretraining: 4.9+/-0.6 mol.kg protein -1.h-1; P=0.76). The active form of PDH was higher after training (main effect, P=0.04), and net muscle glycogenolysis (posttraining: 100+/-16 vs. pretraining: 139+/-11 mmol/kg dry wt; P=0.03) and lactate accumulation (posttraining: 55+/-2 vs. pretraining: 63+/-1 mmol/kg dry wt; P=0.03) during exercise were reduced.liii
- Biopsy samples obtained before and after training revealed similar increases in muscle oxidative capacity, as reflected by the maximal activity of cytochrome c oxidase (COX) and COX subunits II and IV protein content (main effects, P </= 0.05), but COX II and IV mRNAs were unchanged. Training-induced increases in muscle buffering capacity and glycogen content were also similar between groups (main effects, P </= 0.05). Given the large difference in training volume, these data demonstrate that SIT is a time-efficient strategy to induce rapid adaptations in skeletal muscle and exercise performance that are comparable to ET in young active men.liv
- HIIT significantly increased muscle mitochondrial beta-hydroxyacyl-CoA dehydrogenase (15.44 +/- 1.57 and 20.35 +/- 1.40 mmol.min(-1).kg wet mass(-1) before and after training, respectively) and citrate synthase (24.45 +/- 1.89 and 29.31 +/- 1.64 mmol.min(-1).kg wet mass(-1) before and after training, respectively) maximal activities by 32% and 20%, while cytoplasmic hormone-sensitive lipase protein content was not significantly increased. Total muscle plasma membrane fatty acid-binding protein content increased significantly (25%), whereas fatty acid translocase/CD36 content was unaffected after HIIT. In summary, seven sessions of HIITover 2 wk induced marked increases in whole body and skeletal muscle capacity for fatty acid oxidation during exercise in moderately active women.lv
- Muscle oxidative capacity, as reflected by the protein content of cytochrome c oxidase subunit 4 (COX4), increased by approximately 35% after 1 wk of SIT and remained higher compared with Pre, even after 6 wk of detraining (P < 0.05). Muscle GLUT4 content increased after 1 wk of SIT and remained approximately 20% higher compared with baseline during detraining (P < 0.05). The monocarboxylate tranporter (MCT) 4 was higher after 1 and 6 wk of SIT compared with Pre, whereas MCT1 increased after 6 wk of training and remained higher after 1 wk of detraining (P < 0.05). There was no effect of training or detraining on the muscle content of fatty acid translocase (FAT/CD36) or plasma membrane associated fatty acid binding protein (FABPpm) (P > 0.05). We conclude that short-term SIT induces rapid increases in skeletal muscle oxidative capacity but has divergent effects on proteins associated with glucose, lactate, and fatty acid transport.lvi
- Despite these differences, both protocols induced similar increases (P < 0.05) in mitochondrial markers for skeletal muscle CHO (pyruvate dehydrogenase E1alpha protein content) and lipid oxidation (3-hydroxyacyl CoA dehydrogenase maximal activity) and protein content of peroxisome proliferator-activated receptor-gamma coactivator-1alpha.lvii
- In two cases, the course was more rapid (death at 7 and 11 weeks of life) and featured marked cardiac hypertrophy (3- and 4-fold increase in heart weight). This predominantly cardiomyopathic phenotype was associated with compound heterozygosity (E140K with another nonsense mutation) in the SCO2 gene. Polioencephalopathy with neurodegeneration and neuronal drop out was present in all cases with evidence that retinal neurons might be seriously affected too. Involvement of spinal motoneurons together with cytochrome c oxidase deficiency in muscle represents a «double hit» for the skeletal muscle. The mitochondrial population was not found to be significantly increased or structurally altered, with the exception of two compound heterozygotes in which the cardiac mitochondria were increased in number and size
- Posttraining, at rest, hexokinase activity increased in type 1 diabetic subjects; in both groups, citrate synthase activity increased and pyruvate dehydrogenase activity decreasedlviii
- After the IT period, the protein expression of skeletal muscle UCP3 tended to be higher in SET (34 +/- 6 vs. 47 +/- 7 arbitrary units; P = 0.06). Activity of muscle citrate synthase and 3-hydroxyacyl-CoA dehydrogenase, as well as maximal oxygen uptake and 10-km performance time, remained unaltered in both groups. In SET, the capillary-to-fiber ratio was the same before and after the IT period. The present study showed that speed endurance training reduces energy expenditure during submaximal exercise, which is not mediated by lowered mitochondrial UCP3 expressionlix
- Muscle adaptations at rest included the following: (i) increased cytochrome c oxidase IV content (18%) and maximal activities of the mitochondrial enzymes citrate synthase (26%), beta-hydroxyacyl-CoA dehydrogenase (29%), aspartate-amino transferase (26%), and pyruvate dehydrogenase (PDH; 21%); (ii) increased FAT/CD36, FABPpm, GLUT 4, and MCT 1 and 4 transport proteins (14%-30%); and (iii) increased glycogen content (59%). Major adaptations during exercise included the following: (i) reduced glycogenolysis, lactate accumulation, and substrate phosphorylation (0-5 min of TE); (ii) unchanged PDH activation (carbohydrate oxidation; 0-5 min of TE); (iii) ~2-fold greater time during the TE; and (iv) increased fat oxidation at 60% of pre-training VO2 peak.lx
- 30-s sprint runs 3-4 times/wk (SET group n = 12) or a control group (n = 5), which continued the endurance training ( approximately 55 km/wk). For the SET group, the expression of the muscle Na(+)-K(+) pump alpha(2)-subunit was 68% higher (P < 0.05) and the plasma K(+) level was reduced (P < 0.05) during repeated intense running after 9 wklxi
- Interval training increased average V O(2)max (7%; p<0.001) and was associated with greater expression of mitochondrial components, including succinate dehydrogenase, trifunctional protein-alpha and ATP synthase alpha- and beta-chainslxii
- After 12 weeks of training, quadriceps muscle mass and mean muscle fibre area were 9 and 15% larger (p < 0.05) in SO, but unaltered in RU, and in SO, the fraction of FTx fibres was lowered (10.7 +/- 1.8 vs. 17.9 +/- 3.2%). In SO, citrate synthase activity was 10 and 14% higher (p < 0.05) after 4 and 12 weeks, but unaltered in RU.lxiii
- Nuclear abundance of peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) was approximately 25% higher after training (P < 0.05), but total PGC-1alpha protein content remained unchanged. Total SIRT1 content, a proposed activator of PGC-1alpha and mitochondrial biogenesis, was increased by approximately 56% following training (P < 0.05). Training also increased resting muscle glycogen and total GLUT4 protein content (both P < 0.05). This study demonstrates that a practical model of low volume HIT is a potent stimulus for increasing skeletal muscle mitochondrial capacity and improving exercise performance. The results also suggest that increases in SIRT1, nuclear PGC-1alpha, and Tfam may be involved in coordinating mitochondrial adaptations in response to HIT in human skeletal muscle.lxiv
- Fat oxidation during steady-state cycling increased after training in LOW (from 26 ± 2 to 34 ± 2 μmol·kg−¹·min−¹, P < 0.01). Plasma free fatty acid oxidation was similar before and after training in both groups, but muscle-derived triacylglycerol oxidation increased after training in LOW (from 16 ± 1 to 23 ± 1 μmol·kg−¹·min−¹, P < 0.05). Training with low muscle glycogen also increased β-hydroxyacyl-CoA-dehydrogenase protein content (P < 0.01).lxv
- No changes were observed in maximal oxygen consumption, muscle fiber type, capillary supply, citrate synthase and 3-hydroxyacetyl CoA dehydrogenase activities. Lactate dehydrogenase (LDH) activity increased in homogenate (P<0.05) and type IIa fiber pools (9.3%, P<0.05). The change in the latter correlated with an absolute interval training speed (r=0.65; P<0.05). In conclusion, HIIT in trained endurance runners causes no adaptations in muscle oxidative capacity but increased LDH activity, especially in type IIa fibers and in relation to absolute HIIT speed.lxvi
- Six weeks of high-intensity interval training ( approximately 1 h of 10 x 4 min intervals at 90% peak oxygen consumption separated by 2 min rest, 3 days per week) increased maximal activities of mitochondrial enzymes in skeletal muscle by 28% to 36% (citrate synthase, beta-hydroxyacyl-coenzyme A dehydrogenase, and cytochrome c oxidase subunit IV) and PGC-1alpha protein (16%) when measured 4 days after traininglxvii
- welve Thoroughbred horses were used for the analysis. For 18 weeks, all the horses underwent high-intensity training (HIT), with running at 90-110% maximal oxygen consumption (VO2 max ) for 3 min, 5 days week(-1). Thereafter, the horses either underwent detraining for 6 weeks by either 3 min of moderate-intensity training (MIT) at 70% VO2 max, 5 days week(-1) (HIT-MIT group) or stall rest (HIT-SR group). The horses underwent an incremental exercise test, VO2 max was measured and resting muscle samples were obtained from the middle gluteus muscle at 0, 18 and 24 weeks. The content of MCT1 and MCT4 proteins increased after 18 weeks of HIT. At the end of this period, an increase was noted in the citrate synthase activity, while phosphofructokinase activity remained unchanged. After 6 weeks of detraining, all these indexes returned to the pretraining levels in the HIT-SR group. However, in the HIT-MIT group, the increase in the MCT1 protein content and citrate synthase activity was maintained after 6 weeks of MIT, while the MCT4 protein content decreased to the pretraining value. lxviii
- The amount of muscle pyruvate dehydrogenase (17%, P < 0.01) and maximal activity of citrate synthase (12%) and 3-hydroxyacyl-CoA (18%, P < 0.05) were lowered. In addition, the fraction of slow twitch fibers (56% ± 18% vs 47% ± 15%, P < 0.05), Yo-Yo intermittent recovery level 2 test (845 ± 160 vs 654 ± 99 m), and the repeated sprintperformance (33.41 ± 0.96 vs 34.11 ± 0.92 s, P < 0.01) were reduced. For the high-intensity training group, running economy was improved (P < 0.05), and the amount of pyruvate dehydrogenase (17%) and repeated sprint performance (33.44 ± 1.17 vs 32.81 ± 1.01 s) were enhanced (P < 0.05).lxix
- Muscle oxidative capacity, as reflected by the protein content of citrate synthase and cytochrome c oxidase subunit IV, increased by ∼35% after training. The transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1α was increased by ∼56% after training, but the transcriptional corepressor receptor-interacting protein 140 remained unchanged. Glucose transporter protein content increased ∼260%, and insulin sensitivity, on the basis of the insulin sensitivity index homeostasis model assessment, improved by ∼35% after training.lxx
- OX4I2 mRNA expression decreased significantly in Group A and remained unchanged in Group B between T(0) vs. T(2) (-1.7-fold, P = 0.017; -1.0-fold, P = 0.859). PDK4 mRNA expression increased significantly in Group B but not in Group A between T(0) vs. T(1) (3.8-fold, P = 0.039; 1.4-fold, P = 0.591). There were no significant changes in the expression in CKM or COX4I1 mRNA abundance in either group.lxxi
- The drop in phosphocreatine and the increases in glucose-6-phosphate and fructose-6-phosphate after two 80-m sprints were greater in the 10-s group. In conclusion, training with a limited number of repeated short sprints (≤10 s) may be more effective in improving speed maintenance in 200- and 300-m runs when performed with a 1:1 rather than a 1:6 exercise-to-rest ratio. This may be due to a greater activation of glycolysis caused, in part, by the limited resynthesis of phosphocreatine during the very short rest interval.lxxii
- Training increased muscle mitochondrial capacity as evidenced by higher citrate synthase maximal activity (∼20%) and protein content of Complex II 70 kDa subunit (∼37%), Complex III Core 2 protein (∼51%), and Complex IV subunit IV (∼68%, all P < 0.05). Mitofusin 2 (∼71%) and GLUT4 (∼369%) protein content were also higher after training (both P < 0.05). Our findings indicate that low-volume HIT can rapidly improve glucose control and induce adaptations in skeletal muscle that are linked to improved metabolic health in patients with type 2 diabetes.lxxiii
- Acute RSE increased the phosphorylation of acetyl-CoA carboxylase (86%, effect size (ES) = 1.4 ± 0.8, P < 0.001) and Ca calmodulin-dependent protein kinase II (69%, ES = 0.7 ± 0.6). Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α; 208%, ES = 1.5 ± 0.7, P < 0.001) and nuclear respiratory factor 1 (92%, ES = 0.7 ± 0.8) mRNA expression was increased after RSE. Four weeks of training increased the abundance of PGC-1α protein at rest (33%, ES = 0.9 ± 0.7).lxxiv
- All subjects completed the protocol, with ratings of discomfort far less than those reported in studies of traditional NMES. Training induced significant increases in SR Ca(2+) release and citrate synthase activity (~16% and 32%, respectively), but SR Ca(2+) uptake did not change. The percentage of myosin heavy chain IIx isoform was decreased significantly after training. At the whole muscle level, neither central activation nor maximum voluntary isometric contraction force were significantly altered, although isometric force did exhibit a trend toward an increase (~3%, P = 0.055). Surprisingly, the NMES training produced a significant increase in muscle cross-sectional area (~3%, P = 0.04).lxxv
- Compared with CON, higher activities were observed in POST (p < 0.05) only for succinic dehydrogenase (3.32 ± 0.16 mol·(mg protein)(-1)·min(-1) vs. 4.10 ± 0.11 mol·(mg protein)(-1)·min(-1)) and hexokinase (0.73 ± 0.05 mol·(mg protein)(-1)·min(-1) vs. 0.90 ± 0.05mol·(mg protein)(-1)·min(-1)) but not for phosphorylase, phosphofructokinase, and creatine phosphokinase. No differences were found in Na(+),K(+)-ATPase concentration (β(max): 262 ± 36 pmol·(g wet weight)(-1) vs. 275 ± 27 pmol·(g wet weight)(-1)) and the maximal activity of the sarcoplasmic reticulum Ca(2+)-ATPase (98.1 ± 6.1 µmol·(g protein)(-1)·min(-1) vs. 102 ± 3.3 µmol·(g protein)(-1)·min(-1)). Cross-sectional area was lower (p < 0.05) in POST but only for the type IIA fibres (6312 ± 684 μm(2) vs. 5512 ± 335 μm(2)), while the number of capillary counts per fibre and the capillary to fibre area ratio were generally higher (p < 0.05). These findings suggest that elite trained ice hockey players display elevations only in support of glucose-based aerobic metabolism that occur in the absence of alterations in excitation-contraction processes.lxxvi
- Muscle samples collected post mortem from the vastus lateralis and longissimus lumborum of fallow deer (Dama dama) and springbok (Antidorcas marsupialis) were analysed for myosin heavy chain isoform content, citrate synthase, 3-hydroxyacylCoA dehydrogenase, phosphofructokinase, lactate dehydrogenase and creatine kinase activities. Cross-sectional areas, fibre type and oxidative capacities of each fibre type were determined in the vastus lateralis only. The predominant fibre type in both muscle groups and species were type IIX (>50%), with springbok having more type IIX fibres than fallow deer (P<0.05). Overall cross-sectional area was not different between the two species. The metabolic pathway analyses showed high glycolytic and oxidative capacities for both species, but springbok had significantly higher CS activities than fallow deer. Large variation and overlap in oxidative capacities existed within and between the fibre types. Some type IIX fibres presented with oxidative capacities similar to those from type I and IIA fibres. The data suggest that springbok and fallow deer are able sprint at >90 and 46 km h(-1), respectively, partly from having large type IIX fibre contents and high glycolytic capacities. The high oxidative capacities also suggest that these animals may be able to withstand fatigue for long periods of time.lxxvii
- Treatment with CoQ10 resulted in a significant increase in total blood CoQ10 (138%; P = 0.02) and reduced blood CoQ10 (168%; P = 0.02), but did not improve exercise performance (with the exception of selected individuals) or impact oxidative stress. The relationship between the percentage change in total blood CoQ10 and the cycle sprint total work (R(2) = 0.6009) was noted to be moderate to strong. We conclude that treatment with CoQ10 in healthy, exercise-trained subjects increases total and reduced blood CoQ10, but this increase does not translate into improved exercise performance or decreased oxidative stress.lxxviii
- Activities of citrate synthase and β-HAD increased after HIT, whereasphosphofructokinase activity remained unchanged. The PGC-1α and FAT/CD36 protein contents increased after HIT, but plasma lactate concentration and the respiratory exchange ratio decreased after HIT. The plasma free fatty acid concentration increased after HIT, whereas the glucose concentration was not altered. Fructose 1,6-diphosphate, phosphoenolpyruvate, and pyruvate concentrations decreased after HIT.lxxix
- Muscle PGC1-α mRNA expression was attenuated as it increased by 11- and 4- fold (P<0.001) after exercise pre- and post-training, respectively. PGC1-α protein expression increased 1.5 fold (P<0.05) in response to exercise pre-training with no further increases after the post-training exercise bout. RIP140 protein abundance was responsive to acute exercise only (P<0.01). COXIV mRNA (1.6 fold; P<0.01) and COXIV protein expression (1.5 fold; P<0.05) were increased by training but COXIV protein expression was decreased (20%; P<0.01) by acute exercise pre- and post-traininglxxx
- intake of antioxidants (α-lipoic acid, vitamin C, and vitamin E) in a double-blind design. Vastus lateralis muscle biopsies were obtained before, immediately postsprint, and 30 and 120 min postsprint. Performance and muscle metabolism were similar during both sprints. TheNAD(+)-to-NADH.H(+) ratio was similarly reduced (84%) and the AMP-to-ATP ratio was similarly increased (×21-fold) immediately after the sprints. Thr(286) Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) and Thr(172)-AMPKα phosphorylations were increased after the control sprint (with placebo) but not when the sprints were preceded by the ingestion of antioxidants. Ser(485)-AMPKα1/Ser(491)-AMPKα2 phosphorylation, a known inhibitory mechanism of Thr(172)-AMPKα phosphorylation, was increased only with antioxidant ingestion. In conclusion, RNOS play a crucial role in AMPK-mediated signaling after sprint exercise in human skeletal muscle. Antioxidant ingestion 2 h before sprint exercise abrogates the Thr(172)-AMPKα phosphorylation response observed after the ingestion of placebo by reducing CaMKII and increasing Ser(485)-AMPKα1/Ser(491)-AMPKα2 phosphorylation. Sprint performance, muscle metabolism, and AMP-to-ATP and NAD(+)-to-NADH.H(+) ratios are not affected by the acute ingestion of antioxidants.lxxxi
- SIT increased muscle phosphofructokinase activity more in HYP (+59%, P < 0.05) than that in NOR (+17%), whereas citrate synthaseactivity was similar between groups. Compared with the pretest, power outputs corresponding to 4 mmol blood lactate in HYP during MAX nor (+7%) and MAX hyp (+9%) were slightly increased (P < 0.05), whereas values were constant in NOR. V·O 2max in MAX nor and TT performance in TT nor and TT hyp were increased by ≈ 6%-8% (P < 0.05) in either group. The training elevated monocarboxylate transporter 1 protein content by ≈ 70% (P < 0.05). In CON, all measurements were constant throughout the study.lxxxii
- Resting muscle biopsies revealed a training-induced increase in mitochondrial capacity as evidenced by increased maximal activities of citrate synthase and β-hydroxyacyl-CoA dehydrogenase (P ≤ 0.05). There was no change in insulin sensitivity, although change in insulin area under the curve was correlated with change in abdominal percent fat (r = 0.54, P ≤ 0.05).lxxxiii
- Skeletal muscle respiratory capacities increased, most likely as a result of an expansion of skeletal muscle mitochondria (∼20%, P = 0.026), as assessed by cytochrome c oxidase activity. Skeletal muscle deoxygenation also increased while maximal cardiac output, total hemoglobin, plasma volume, total blood volume, and relative measures of peripheral fatigue resistance were all unaltered with training. These results suggest that increases in mitochondrial content following six HIT sessions may facilitate improvements in respiratory capacity and oxygen extraction, and ultimately are responsible for the improvements in maximal whole body exercise capacity and endurance performance in previously untrained individuals.lxxxiv
- Density of GLUT4 clusters was higher at the fibre periphery especially in perinuclear regions. A less dense punctate distribution was seen in the rest of the muscle fibre. Total GLUT4 fluorescence intensity increased in type I and type II fibres following both ET and SIT. Large GLUT4 clusters increased in number and size in both type I and type II fibres, while the smaller clusters increased in size. The greatest increases in GLUT4 fluorescence intensity occurred within the 1 μm layer immediately adjacent to the PM. The increase in peripheral localisation and protein content of GLUT4 following ET and SIT is likely to contribute to the improvements in glucose homeostasis observed after both training modes.lxxxv
- Skeletal muscle biopsy samples obtained before and 72 h after training revealed increased maximal activity of citrate synthase and protein content of cytochrome oxidase 4 (p<0.01, main effect), while the maximal activity of β-hydroxy acyl CoA dehydrogenase increased in men onlylxxxvi
- Only the ES group increased in leg strength (+19%, P < 0.01), sprint peak power (+5%, P < 0.05), and short-term endurance (+9%, P < 0.01). In contrast, only the E group increased in muscle citrate synthase activity (+11%, P = 0.06), lactate threshold intensity (+3%, P < 0.05), and long-term endurance performance (+4%, P < 0.05). Content of mitochondrial proteins and cycling economy was not affected by traininglxxxvii
- HIT training improved peak power and time to fatigue. Increases in absolute oxidative phosphorylation (OXPHOS) capacities and CS activity were observed, but not in the ratio of CCO to the electron transport system (CCO/ETS), the respiratory control ratios (RCR-1 and RCR-2) or mitochondrial-associated protein expression. Specific increases in OXPHOS flux were not apparent after normalization to CS, indicating that gross changes mainly resulted from increased mitochondrial mass.lxxxviii
- No change was observed in maximal mitochondrial respiration (VMAX) or Citrate Synthase (CS) activity within or between interventions. Basal respiration (V0) increased after 1-AIT (p=0.029) and 4-AIT (p=0.022), with no significant change after MCT.lxxxix
- Plasma volume and hemoglobin mass increased after 1HIIT only (5.6% and 6.5%); however, no group differences were found. All groups increased CS activity (4HIIT, 35%; 1HIIT, 35%; MICT, 56%), with no group differences. Arterial inflow (15.7%) and venous outflow (22.7%) decreased after MICT, but there were no group differences.xc
- Peak 5-min game distance faster than 21 km h(-1) was related to the Na(+)-K(+) ATPase subunit (α1, α2, β1 and FXYD1) protein levels (r = 0.54-0.70), while Yo-Yo IR2 performance explained 40 % of the variance in high-intensity game distance. Total and 1-min peak sprint distance correlated to myosin heavy chain II/I ratio (MHCII/I ratio) and sarcoendoplasmic reticulum Ca(2+) ATPase isoform-1 (SERCA1) protein (r = 0.56-0.86), while phosphofructokinase (PFK) maximal activity also correlated to total sprint distance (r = 0.46). xci
- Efficient adaptation to exercise training requires optimal exposure to pulses of RONS. Inappropriate training stimulus may lead to excessive RONS formation, oxidative inactivation of AMPK and reduced adaptation or even maladaptation. Theoretically, exercise programs should be designed taking into account the intrinsic properties of different skeletal muscles, the specific RONS induction and the subsequent signaling responses.xcii
- Skeletal muscle mitochondrial content also increased similarly after SIT and MICT, as primarily reflected by the maximal activity of citrate synthase (CS; P<0.001). The corresponding changes in the control group were small for VO2peak (p = 0.99), CSI (p = 0.63) and CS (p = 0.97).xciii
- кинетика и % ммв http://jap.physiology.org/content/81/4/1642http://jap.physiology.org/content/114/11/1527http://ajpendo.physiology.org/content/292/1/E107MkArdle http://www.ncbi.nlm.nih.gov/pubmed/20683610
iWee Kian Yeo et al., “Skeletal Muscle Adaptation and Performance Responses to Once a Day versus Twice Every Second Day Endurance Training Regimens,” Journal of Applied Physiology (Bethesda, Md.: 1985) 105, no. 5 (November 2008): 1462–70, doi:10.1152/japplphysiol.90882.2008. http://jap.physiology.org/content/105/5/1462.long
iiInge D. Wijnberg et al., “(Over)training Effects on Quantitative Electromyography and Muscle Enzyme Activities in Standardbred Horses,” Journal of Applied Physiology (Bethesda, Md.: 1985) 105, no. 6 (December 2008): 1746–53, doi:10.1152/japplphysiol.01272.2007. http://jap.physiology.org/content/105/6/1746.long
iiiVernon G. Coffey et al., “Effect of Consecutive Repeated Sprint and Resistance Exercise Bouts on Acute Adaptive Responses in Human Skeletal Muscle,” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 297, no. 5 (November 2009): R1441–51, doi:10.1152/ajpregu.00351.2009. http://ajpregu.physiology.org/content/297/5/R1441.long
ivNiklas Psilander et al., “Mitochondrial Gene Expression in Elite Cyclists: Effects of High-Intensity Interval Exercise,” European Journal of Applied Physiology 110, no. 3 (October 2010): 597–606, doi:10.1007/s00421-010-1544-1. http://dx.doi.org.sci-hub.cc/10.1007/s00421-010-1544-1
vChristopher G. R. Perry et al., “Repeated Transient mRNA Bursts Precede Increases in Transcriptional and Mitochondrial Proteins during Training in Human Skeletal Muscle,” The Journal of Physiology 588, no. Pt 23 (December 1, 2010): 4795–4810, doi:10.1113/jphysiol.2010.199448. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3010147/
viAndrew J. R. Cochran et al., “Intermittent and Continuous High-Intensity Exercise Training Induce Similar Acute but Different Chronic Muscle Adaptations,” Experimental Physiology 99, no. 5 (May 1, 2014): 782–91, doi:10.1113/expphysiol.2013.077453. http://onlinelibrary.wiley.com/doi/10.1113/expphysiol.2013.077453/abstract;jsessionid=EF32DDCD78D916314A5551AA912CC0E8.f03t01
viiPeter M. Christensen et al., “A Short Period of High-Intensity Interval Training Improves Skeletal Muscle Mitochondrial Function and Pulmonary Oxygen Uptake Kinetics,” Journal of Applied Physiology (Bethesda, Md.: 1985), February 4, 2016, jap.00115.2015, doi:10.1152/japplphysiol.00115.2015.
viiiAndrew J. Cochran et al., “Manipulating Carbohydrate Availability Between Twice-Daily Sessions of High-Intensity Interval Training Over 2 Weeks Improves Time-Trial Performance,” International Journal of Sport Nutrition and Exercise Metabolism 25, no. 5 (October 2015): 463–70, doi:10.1123/ijsnem.2014-0263. http://www.ncbi.nlm.nih.gov/pubmed/?term=25811132
ixPeter M. Christensen et al., “Unchanged Content of Oxidative Enzymes in Fast-Twitch Muscle Fibers and V˙O2 Kinetics after Intensified Training in Trained Cyclists,” Physiological Reports 3, no. 7 (July 2015), doi:10.14814/phy2.12428. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4552518/
xDaisuke Hoshino et al., “Effects of Decreased Lactate Accumulation after Dichloroacetate Administration on Exercise Training-Induced Mitochondrial Adaptations in Mouse Skeletal Muscle,” Physiological Reports 3, no. 9 (September 2015), doi:10.14814/phy2.12555. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600395/
xiCesare Granata et al., “Training Intensity Modulates Changes in PGC-1α and p53 Protein Content and Mitochondrial Respiration, but Not Markers of Mitochondrial Content in Human Skeletal Muscle,” FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 30, no. 2 (February 2016): 959–70, doi:10.1096/fj.15-276907. http://sci-hub.cc/10.1096/fj.15-276907
xiiFilip J. Larsen et al., “High-Intensity Sprint Training Inhibits Mitochondrial Respiration through Aconitase Inactivation,” FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 30, no. 1 (January 2016): 417–27, doi:10.1096/fj.15-276857. http://sci-hub.cc/10.1096/fj.15-276857
Далее скучное и малополезное
xiiiA. Thorstensson, B. Sjödin, and J. Karlsson, “Enzyme Activities and Muscle Strength after ‘Sprint Training’ in Man,” Acta Physiologica Scandinavica 94, no. 3 (July 1975): 313–18, doi:10.1111/j.1748-1716.1975.tb05891.x.
xivD. L. Costill et al., “Skeletal Muscle Enzymes and Fiber Composition in Male and Female Track Athletes,” Journal of Applied Physiology 40, no. 2 (February 1976): 149–54.
xvB. Saltin et al., “The Nature of the Training Response; Peripheral and Central Adaptations of One-Legged Exercise,” Acta Physiologica Scandinavica 96, no. 3 (March 1976): 289–305, doi:10.1111/j.1748-1716.1976.tb10200.x.
xviE. R. Burke et al., “Characteristics of Skeletal Muscle in Competitive Cyclists,” Medicine and Science in Sports 9, no. 2 (1977): 109–12.
xviiM. Fournier et al., “Skeletal Muscle Adaptation in Adolescent Boys: Sprint and Endurance Training and Detraining,” Medicine and Science in Sports and Exercise 14, no. 6 (1982): 453–56.
xviiiA. D. Roberts, R. Billeter, and H. Howald, “Anaerobic Muscle Enzyme Changes after Interval Training,” International Journal of Sports Medicine 3, no. 1 (February 1982): 18–21, doi:10.1055/s-2008-1026055.
xixJ. A. Simoneau et al., “Inheritance of Human Skeletal Muscle and Anaerobic Capacity Adaptation to High-Intensity Intermittent Training,” International Journal of Sports Medicine 7, no. 3 (June 1986): 167–71, doi:10.1055/s-2008-1025756.
xxM. E. Cheetham et al., “Human Muscle Metabolism during Sprint Running,” Journal of Applied Physiology (Bethesda, Md.: 1985) 61, no. 1 (July 1986): 54–60.
xxiE. Macková et al., “Skeletal Muscle Characteristics of Sprint Cyclists and Nonathletes,” International Journal of Sports Medicine 7, no. 5 (October 1986): 295–97.
xxiiC. V. Foster and R. C. Harris, “Formation of Acetylcarnitine in Muscle of Horse during High Intensity Exercise,” European Journal of Applied Physiology and Occupational Physiology 56, no. 6 (1987): 639–42.
xxiiiG. P. Dobson, W. S. Parkhouse, and P. W. Hochachka, “Regulation of Anaerobic ATP-Generating Pathways in Trout Fast-Twitch Skeletal Muscle,” The American Journal of Physiology 253, no. 1 Pt 2 (July 1987): R186–94.
xxivI. Jacobs et al., “Sprint Training Effects on Muscle Myoglobin, Enzymes, Fiber Types, and Blood Lactate,” Medicine and Science in Sports and Exercise 19, no. 4 (August 1987): 368–74.
xxvD. L. Costill et al., “Effects of Repeated Days of Intensified Training on Muscle Glycogen and Swimming Performance,” Medicine and Science in Sports and Exercise 20, no. 3 (June 1988): 249–54.
xxviM. Gottlieb et al., “Effects of a Draft-Loaded Interval-Training Program on Skeletal Muscle in the Horse,” Journal of Applied Physiology (Bethesda, Md.: 1985) 67, no. 2 (August 1989): 570–77.
xxviiJ. Cadefau et al., “Biochemical and Histochemical Adaptation to Sprint Training in Young Athletes,” Acta Physiologica Scandinavica 140, no. 3 (November 1990): 341–51, doi:10.1111/j.1748-1716.1990.tb09008.x.
xxviiiE. M. Gorostiaga et al., “Uniqueness of Interval and Continuous Training at the Same Maintained Exercise Intensity,” European Journal of Applied Physiology and Occupational Physiology 63, no. 2 (1991): 101–7.
xxixB. Shepley et al., “Physiological Effects of Tapering in Highly Trained Athletes,” Journal of Applied Physiology (Bethesda, Md.: 1985) 72, no. 2 (February 1992): 706–11.
xxxM. T. Linossier et al., “Ergometric and Metabolic Adaptation to a 5-S Sprint Training Programme,” European Journal of Applied Physiology and Occupational Physiology 67, no. 5 (1993): 408–14.
xxxiJ. Bangsbo, L. Michalsik, and A. Petersen, “Accumulated O2 Deficit during Intense Exercise and Muscle Characteristics of Elite Athletes,” International Journal of Sports Medicine 14, no. 4 (May 1993): 207–13, doi:10.1055/s-2007-1021165.
xxxiiM. Esbjörnsson et al., “Fast Twitch Fibres May Predict Anaerobic Performance in Both Females and Males,” International Journal of Sports Medicine 14, no. 5 (July 1993): 257–63, doi:10.1055/s-2007-1021174.
xxxiiiY. Hellsten-Westing et al., “The Effect of High-Intensity Training on Purine Metabolism in Man,” Acta Physiologica Scandinavica 149, no. 4 (December 1993): 405–12, doi:10.1111/j.1748-1716.1993.tb09636.x.
xxxivH. L. Olesen et al., “Maximal Oxygen Deficit of Sprint and Middle Distance Runners,” European Journal of Applied Physiology and Occupational Physiology 69, no. 2 (1994): 140–46.
xxxvB. Norman et al., “AMP Deaminase in Skeletal Muscle of Healthy Males Quantitatively Determined by New Assay,” Acta Physiologica Scandinavica 150, no. 4 (April 1994): 397–403, doi:10.1111/j.1748-1716.1994.tb09704.x.
xxxviM. Esbjörnsson Liljedahl et al., “Different Responses of Skeletal Muscle Following Sprint Training in Men and Women,” European Journal of Applied Physiology and Occupational Physiology 74, no. 4 (1996): 375–83.
xxxviiY. Hellsten, F. S. Apple, and B. Sjödin, “Effect of Sprint Cycle Training on Activities of Antioxidant Enzymes in Human Skeletal Muscle,” Journal of Applied Physiology (Bethesda, Md.: 1985) 81, no. 4 (October 1996): 1484–87.
xxxviiiA. R. Weston et al., “Skeletal Muscle Buffering Capacity and Endurance Performance after High-Intensity Interval Training by Well-Trained Cyclists,” European Journal of Applied Physiology and Occupational Physiology 75, no. 1 (1997): 7–13.
xxxixM. T. Linossier et al., “Enzyme Adaptations of Human Skeletal Muscle during Bicycle Short-Sprint Training and Detraining,” Acta Physiologica Scandinavica 161, no. 4 (December 1997): 439–45, doi:10.1046/j.1365-201X.1997.00244.x.
xlJ. D. MacDougall et al., “Muscle Performance and Enzymatic Adaptations to Sprint Interval Training,” Journal of Applied Physiology (Bethesda, Md.: 1985) 84, no. 6 (June 1998): 2138–42.
xliB. Dawson et al., “Changes in Performance, Muscle Metabolites, Enzymes and Fibre Types after Short Sprint Training,” European Journal of Applied Physiology and Occupational Physiology 78, no. 2 (July 1998): 163–69, doi:10.1007/s004210050402.
xliiR. A. Howlett, G. J. Heigenhauser, and L. L. Spriet, “Skeletal Muscle Metabolism during High-Intensity Sprint Exercise Is Unaffected by Dichloroacetate or Acetate Infusion,” Journal of Applied Physiology (Bethesda, Md.: 1985) 87, no. 5 (November 1999): 1747–51.
xliiiM. T. Linossier et al., “Effect of Hyperoxia on Aerobic and Anaerobic Performances and Muscle Metabolism during Maximal Cycling Exercise,” Acta Physiologica Scandinavica 168, no. 3 (March 2000): 403–11, doi:10.1046/j.1365-201x.2000.00648.x.
xlivJ. Parra et al., “The Distribution of Rest Periods Affects Performance and Adaptations of Energy Metabolism Induced by High-Intensity Training in Human Muscle,” Acta Physiologica Scandinavica 169, no. 2 (June 2000): 157–65, doi:10.1046/j.1365-201x.2000.00730.x. https://www.researchgate.net/publication/12469173_The_distribution_of_rest_periods_affects_performance_and_adaptations_of_energy_metabolism_induced_by_high-intensity_training_in_human_muscle
xlvG. Rodas et al., “A Short Training Programme for the Rapid Improvement of Both Aerobic and Anaerobic Metabolism,” European Journal of Applied Physiology 82, no. 5–6 (August 2000): 480–86, doi:10.1007/s004210000223.
xlviZ. P. Chen et al., “AMPK Signaling in Contracting Human Skeletal Muscle: Acetyl-CoA Carboxylase and NO Synthase Phosphorylation,” American Journal of Physiology. Endocrinology and Metabolism 279, no. 5 (November 2000): E1202–6.
xlviiS. A. Clark et al., “Intensified Exercise Training Does Not Alter AMPK Signaling in Human Skeletal Muscle,” American Journal of Physiology. Endocrinology and Metabolism 286, no. 5 (May 2004): E737–43, doi:10.1152/ajpendo.00462.2003.
xlviiiC. Thomas et al., “Relationships between Maximal Muscle Oxidative Capacity and Blood Lactate Removal after Supramaximal Exercise and Fatigue Indexes in Humans,” Journal of Applied Physiology (Bethesda, Md.: 1985) 97, no. 6 (December 2004): 2132–38, doi:10.1152/japplphysiol.00387.2004.
xlixC. Barnett et al., “Muscle Metabolism during Sprint Exercise in Man: Influence of Sprint Training,” Journal of Science and Medicine in Sport / Sports Medicine Australia 7, no. 3 (September 2004): 314–22.
lC. Thomas et al., “Monocarboxylate Transporters, Blood Lactate Removal after Supramaximal Exercise, and Fatigue Indexes in Humans,” Journal of Applied Physiology (Bethesda, Md.: 1985) 98, no. 3 (March 2005): 804–9, doi:10.1152/japplphysiol.01057.2004.
liKirsten A. Burgomaster et al., “Six Sessions of Sprint Interval Training Increases Muscle Oxidative Potential and Cycle Endurance Capacity in Humans,” Journal of Applied Physiology (Bethesda, Md.: 1985) 98, no. 6 (June 2005): 1985–90, doi:10.1152/japplphysiol.01095.2004.
liiFabien A. Basset et al., “Effects of Short-Term Normobaric Hypoxia on Haematology, Muscle Phenotypes and Physical Performance in Highly Trained Athletes,” Experimental Physiology 91, no. 2 (March 2006): 391–402, doi:10.1113/expphysiol.2005.031682.
liiiKirsten A. Burgomaster, George J. F. Heigenhauser, and Martin J. Gibala, “Effect of Short-Term Sprint Interval Training on Human Skeletal Muscle Carbohydrate Metabolism during Exercise and Time-Trial Performance,” Journal of Applied Physiology (Bethesda, Md.: 1985) 100, no. 6 (June 2006): 2041–47, doi:10.1152/japplphysiol.01220.2005.
livMartin J. Gibala et al., “Short-Term Sprint Interval versus Traditional Endurance Training: Similar Initial Adaptations in Human Skeletal Muscle and Exercise Performance,” The Journal of Physiology 575, no. Pt 3 (September 15, 2006): 901–11, doi:10.1113/jphysiol.2006.112094.
lvJason L. Talanian et al., “Two Weeks of High-Intensity Aerobic Interval Training Increases the Capacity for Fat Oxidation during Exercise in Women,” Journal of Applied Physiology (Bethesda, Md.: 1985) 102, no. 4 (April 2007): 1439–47, doi:10.1152/japplphysiol.01098.2006.
lviKirsten A. Burgomaster et al., “Divergent Response of Metabolite Transport Proteins in Human Skeletal Muscle after Sprint Interval Training and Detraining,” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 292, no. 5 (May 2007): R1970–76, doi:10.1152/ajpregu.00503.2006.
lviiKirsten A. Burgomaster et al., “Similar Metabolic Adaptations during Exercise after Low Volume Sprint Interval and Traditional Endurance Training in Humans,” The Journal of Physiology 586, no. 1 (January 1, 2008): 151–60, doi:10.1113/jphysiol.2007.142109.
lviiiAlison R. Harmer et al., “Sprint Training Increases Muscle Oxidative Metabolism during High-Intensity Exercise in Patients with Type 1 Diabetes,” Diabetes Care 31, no. 11 (November 2008): 2097–2102, doi:10.2337/dc08-0329.
lixF. Marcello Iaia et al., “Four Weeks of Speed Endurance Training Reduces Energy Expenditure during Exercise and Maintains Muscle Oxidative Capacity despite a Reduction in Training Volume,” Journal of Applied Physiology (Bethesda, Md.: 1985) 106, no. 1 (January 2009): 73–80, doi:10.1152/japplphysiol.90676.2008.
lxChristopher G. R. Perry et al., “High-Intensity Aerobic Interval Training Increases Fat and Carbohydrate Metabolic Capacities in Human Skeletal Muscle,” Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquée, Nutrition Et Métabolisme 33, no. 6 (December 2008): 1112–23, doi:10.1139/H08-097.
lxiJens Bangsbo et al., “Reduced Volume and Increased Training Intensity Elevate Muscle Na+-K+ Pump alpha2-Subunit Expression as Well as Short- and Long-Term Work Capacity in Humans,” Journal of Applied Physiology (Bethesda, Md.: 1985) 107, no. 6 (December 2009): 1771–80, doi:10.1152/japplphysiol.00358.2009.
lxiiKathryn V. Holloway et al., “Proteomic Investigation of Changes in Human Vastus Lateralis Muscle in Response to Interval-Exercise Training,” Proteomics 9, no. 22 (November 2009): 5155–74, doi:10.1002/pmic.200900068.
lxiiiPeter Krustrup et al., “Muscle Adaptations and Performance Enhancements of Soccer Training for Untrained Men,” European Journal of Applied Physiology 108, no. 6 (April 2010): 1247–58, doi:10.1007/s00421-009-1319-8.
lxivJonathan P. Little et al., “A Practical Model of Low-Volume High-Intensity Interval Training Induces Mitochondrial Biogenesis in Human Skeletal Muscle: Potential Mechanisms,” The Journal of Physiology 588, no. Pt 6 (March 15, 2010): 1011–22, doi:10.1113/jphysiol.2009.181743.
lxvCarl J. Hulston et al., “Training with Low Muscle Glycogen Enhances Fat Metabolism in Well-Trained Cyclists,” Medicine and Science in Sports and Exercise 42, no. 11 (November 2010): 2046–55, doi:10.1249/MSS.0b013e3181dd5070.
lxviT. A. Kohn, B. Essén-Gustavsson, and K. H. Myburgh, “Specific Muscle Adaptations in Type II Fibers after High-Intensity Interval Training of Well-Trained Runners,” Scandinavian Journal of Medicine & Science in Sports 21, no. 6 (December 2011): 765–72, doi:10.1111/j.1600-0838.2010.01136.x.
lxviiBrendon J. Gurd et al., “High-Intensity Interval Training Increases SIRT1 Activity in Human Skeletal Muscle,” Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquée, Nutrition Et Métabolisme 35, no. 3 (June 2010): 350–57, doi:10.1139/H10-030.
lxviiiYu Kitaoka et al., “Effect of Training and Detraining on Monocarboxylate Transporter (MCT) 1 and MCT4 in Thoroughbred Horses,” Experimental Physiology 96, no. 3 (March 2011): 348–55, doi:10.1113/expphysiol.2010.055483.
lxixPeter M. Christensen et al., “VO2 Kinetics and Performance in Soccer Players after Intense Training and Inactivity,” Medicine and Science in Sports and Exercise 43, no. 9 (September 2011): 1716–24, doi:10.1249/MSS.0b013e318211c01a.
lxxMelanie S. Hood et al., “Low-Volume Interval Training Improves Muscle Oxidative Capacity in Sedentary Adults,” Medicine and Science in Sports and Exercise 43, no. 10 (October 2011): 1849–56, doi:10.1249/MSS.0b013e3182199834.
lxxiE. W. Hill et al., “Moderate and High Intensity Sprint Exercise Induce Differential Responses in COX4I2 and PDK4 Gene Expression in Thoroughbred Horse Skeletal Muscle,” Equine Veterinary Journal. Supplement, no. 38 (November 2010): 576–81, doi:10.1111/j.2042-3306.2010.00206.x.
lxxiiPloutarchos Saraslanidis et al., “Muscle Metabolism and Performance Improvement after Two Training Programmes of Sprint Running Differing in Rest Interval Duration,” Journal of Sports Sciences 29, no. 11 (August 2011): 1167–74, doi:10.1080/02640414.2011.583672.
lxxiiiJonathan P. Little et al., “Low-Volume High-Intensity Interval Training Reduces Hyperglycemia and Increases Muscle Mitochondrial Capacity in Patients with Type 2 Diabetes,” Journal of Applied Physiology (Bethesda, Md.: 1985) 111, no. 6 (December 2011): 1554–60, doi:10.1152/japplphysiol.00921.2011.
lxxivFabio R. Serpiello et al., “Repeated Sprints Alter Signaling Related to Mitochondrial Biogenesis in Humans,” Medicine and Science in Sports and Exercise 44, no. 5 (May 2012): 827–34, doi:10.1249/MSS.0b013e318240067e.
lxxvDavid W. Russ et al., “Development of a Neuromuscular Electrical Stimulation Protocol for Sprint Training,” Medicine and Science in Sports and Exercise 44, no. 9 (September 2012): 1810–19, doi:10.1249/MSS.0b013e31825423f1.
lxxviHoward J. Green et al., “Muscle Cellular Properties in the Ice Hockey Player: A Model for Investigating Overtraining?,” Canadian Journal of Physiology and Pharmacology 90, no. 5 (May 2012): 567–78, doi:10.1139/y2012-017.
lxxviiJennifer Wendy Curry et al., “High Oxidative Capacity and Type IIx Fibre Content in Springbok and Fallow Deer Skeletal Muscle Suggest Fast Sprinters with a Resistance to Fatigue,” The Journal of Experimental Biology 215, no. Pt 22 (November 15, 2012): 3997–4005, doi:10.1242/jeb.073684.
lxxviiiRichard J. Bloomer et al., “Impact of Oral Ubiquinol on Blood Oxidative Stress and Exercise Performance,” Oxidative Medicine and Cellular Longevity 2012 (2012): 465020, doi:10.1155/2012/465020.
lxxixYu Kitaoka et al., “Effects of High-Intensity Training on Lipid Metabolism in Thoroughbreds,” American Journal of Veterinary Research 73, no. 11 (November 2012): 1813–18, doi:10.2460/ajvr.73.11.1813.
lxxxNigel K. Stepto et al., “Short-Term Intensified Cycle Training Alters Acute and Chronic Responses of PGC1α and Cytochrome C Oxidase IV to Exercise in Human Skeletal Muscle,” PloS One 7, no. 12 (2012): e53080, doi:10.1371/journal.pone.0053080.
lxxxiDavid Morales-Alamo et al., “Critical Role for Free Radicals on Sprint Exercise-Induced CaMKII and AMPKα Phosphorylation in Human Skeletal Muscle,” Journal of Applied Physiology (Bethesda, Md.: 1985) 114, no. 5 (March 1, 2013): 566–77, doi:10.1152/japplphysiol.01246.2012.
lxxxiiJoke Puype et al., “Sprint Interval Training in Hypoxia Stimulates Glycolytic Enzyme Activity,” Medicine and Science in Sports and Exercise 45, no. 11 (November 2013): 2166–74, doi:10.1249/MSS.0b013e31829734ae.
lxxxiiiJenna B. Gillen et al., “Interval Training in the Fed or Fasted State Improves Body Composition and Muscle Oxidative Capacity in Overweight Women,” Obesity (Silver Spring, Md.) 21, no. 11 (November 2013): 2249–55, doi:10.1002/oby.20379.
lxxxivRobert Acton Jacobs et al., “Improvements in Exercise Performance with High-Intensity Interval Training Coincide with an Increase in Skeletal Muscle Mitochondrial Content and Function,” Journal of Applied Physiology (Bethesda, Md.: 1985) 115, no. 6 (September 2013): 785–93, doi:10.1152/japplphysiol.00445.2013.
lxxxvHelen Bradley et al., “Quantitative Immunofluorescence Microscopy of Subcellular GLUT4 Distribution in Human Skeletal Muscle: Effects of Endurance and Sprint Interval Training,” Physiological Reports 2, no. 7 (July 1, 2014), doi:10.14814/phy2.12085.
lxxxviJenna B. Gillen et al., “Three Minutes of All-out Intermittent Exercise per Week Increases Skeletal Muscle Oxidative Capacity and Improves Cardiometabolic Health,” PloS One 9, no. 11 (2014): e111489, doi:10.1371/journal.pone.0111489.
lxxxviiN. Psilander et al., “Adding Strength to Endurance Training Does Not Enhance Aerobic Capacity in Cyclists,” Scandinavian Journal of Medicine & Science in Sports 25, no. 4 (August 2015): e353–59, doi:10.1111/sms.12338.
lxxxviiiGrace Vincent et al., “Changes in Mitochondrial Function and Mitochondria Associated Protein Expression in Response to 2-Weeks of High Intensity Interval Training,” Frontiers in Physiology 6 (2015): 51, doi:10.3389/fphys.2015.00051.
lxxxixNina Zisko et al., “Effect of Change in VO2max on Daily Total Energy Expenditure in a Cohort of Norwegian Men: A Randomized Pilot Study,” The Open Cardiovascular Medicine Journal 9 (2015): 50–57, doi:10.2174/1874192401509010050.
xcFredrik Hjulstad Bækkerud et al., “Comparison of Three Popular Exercise Modalities on V˙O2max in Overweight and Obese,” Medicine and Science in Sports and Exercise 48, no. 3 (March 2016): 491–98, doi:10.1249/MSS.0000000000000777.
xciMagni Mohr et al., “Muscle Variables of Importance for Physiological Performance in Competitive Football,” European Journal of Applied Physiology 116, no. 2 (February 2016): 251–62, doi:10.1007/s00421-015-3274-x.
xciiDavid Morales-Alamo and Jose A. L. Calbet, “AMPK Signaling in Skeletal Muscle during Exercise: Role of Reactive Oxygen and Nitrogen Species,” Free Radical Biology & Medicine, January 19, 2016, doi:10.1016/j.freeradbiomed.2016.01.012.
xciiiJenna B. Gillen et al., “Twelve Weeks of Sprint Interval Training Improves Indices of Cardiometabolic Health Similar to Traditional Endurance Training despite a Five-Fold Lower Exercise Volume and Time Commitment,” PloS One 11, no. 4 (2016): e0154075, doi:10.1371/journal.pone.0154075.